
Image

| SCEMBLIX is a NOVEL STAMP inhibitor with a different mechanism of action specifically targeting the ABL myristoyl pocket1 |
Dive In: See what’s new
SCEMBLIX targets a specific site on BCR::ABL1 - the myristoyl pocket.2
The myristoyl pocket is a distinct site of the kinase domain, normally occupied by the myristoylated N-terminal of ABL1 in people who do not have CML.3
By binding to the myristoyl pocket, SCEMBLIX allows for specificity in treating CML – targeting BCR :: ABL14
Image

| A different way to treat CML: Scemblix |
Scemblix is a new treatment for later lines of CML1, and it's the first in class approved treatment of its kind.2 | Image
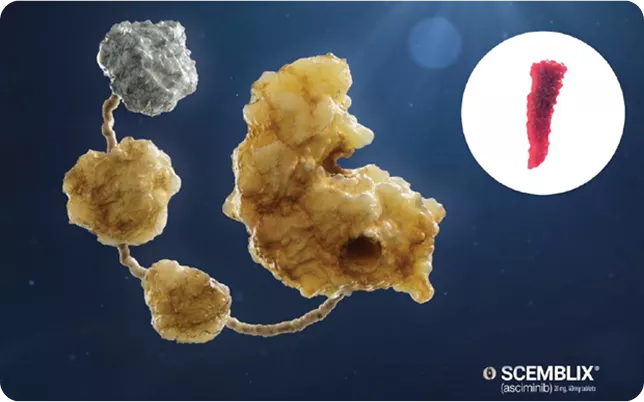
|
Image
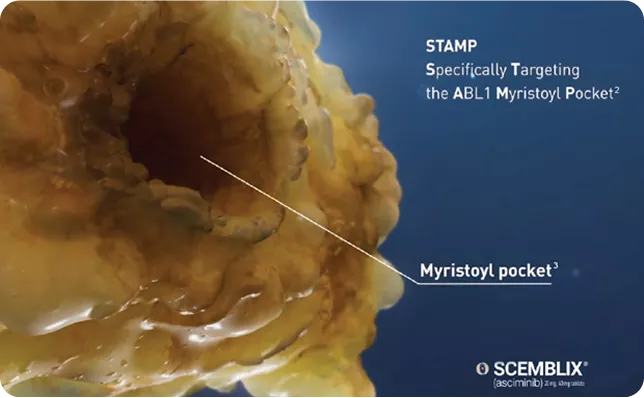
| It's a STAMP inhibitor meaning it specifically targets the ABL1 Myristoyl Pocket.2 |
Scemblix specifically targets ABL1 in the Myristoyl pocket, which is distinct from the ATP binding site.5 | Image
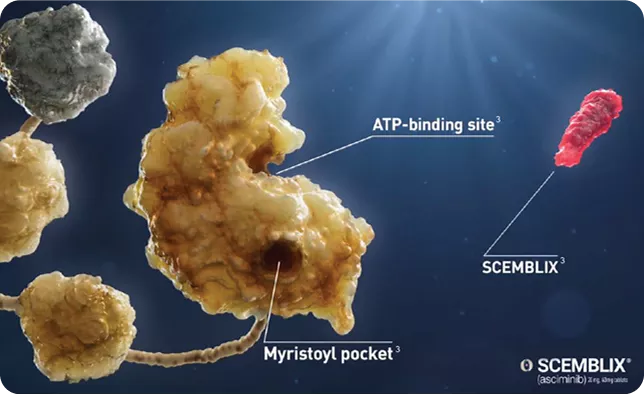
|
Image
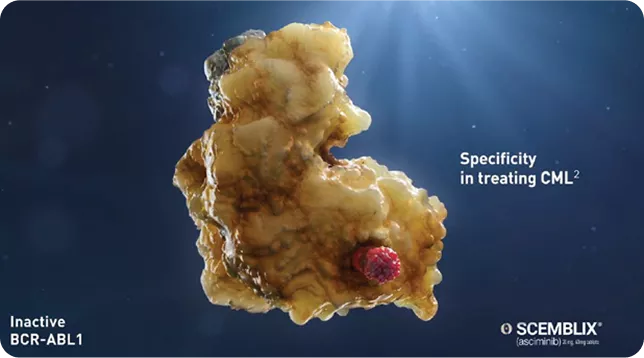
| When Scemblix binds to the Myristoyl pocket, it acts as the missing part of the kinase and inhibits the activity of BCR-ABL1.1
|
Approach CML differently with Scemblix2, the first in class approved STAMP inhibitor specifically targeting the ABL1 Myristoyl pocket and CML.1 | Image

|
CML, chronic myeloid leukemia; ABL 1, Abelson protein tyrosine kinase 1; TKI, Tyrosine Kinase Inhibitor; BCR, breakpoint cluster region; IS, International Scale; STAMP, specifically targeting the ABL1 myristoyl pocket; MMR, Major molecular response; MR4, BCR::ABL1IS 0.01%
For Scemblix 20mg and 40mg Abbreviated Prescription Information
For Scemblix 20mg and 40mg Abbreviated Prescription Information
References
Food and Drug administration (FDA). Scemblix MULTI-DISCIPLINE REVIEW. available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/215358Orig1s000,Orig2s000MultidisciplineR.pdf . Last accessed 16/6/2025
Choi EJ. Asciminib: the first-in-class allosteric inhibitor of BCR:: ABL1 kinase. Blood research. 2023 Apr 30;58(S1):S29-36.
Scemblix Egyptian Drug Authority (EDA) Approved leaflet 05/05/2025.
Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, Turkina A, Kim DW, Apperley JF, Abdo A, Fogliatto LM. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood, The Journal of the American Society of Hematology. 2021 Nov 25;138(21):2031-41.
Réa D, Hughes TP. Development of asciminib, a novel allosteric inhibitor of BCR-ABL1. Critical Reviews in Oncology/Hematology. 2022 Mar 1;171:103580.
Approved by Egyptian Drug Authority: HF0424OA4707/092025. Invalidation date: 18/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
HF0424OA4707/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |

