
Delivering a compelling combination between proven efficacy and established tolerability.1
A TARGETED APPROACH AGAINST CML2
SCEMBLIX is a NOVEL STAMP inhibitor with a different mechanism of action specifically targeting the ABL myristoyl pocket3
Do your CML patients need a different approach?

Early Failure on 2nd line TKI
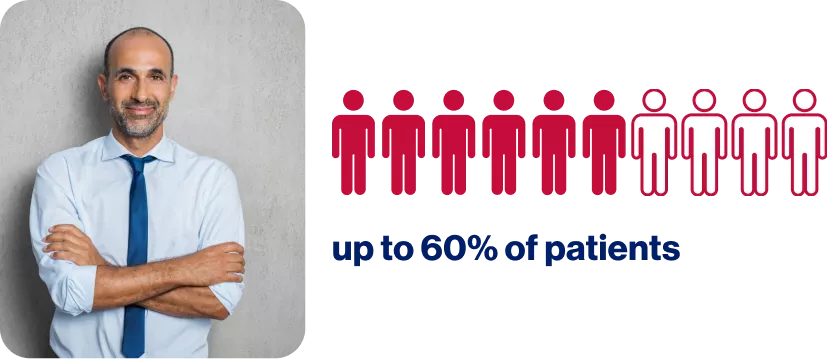
Failure to achieve early response can prevent patients from reaching their treatment goals7
Discontinued 2nd line TKI mainly due to resistance4
A patient having BCR-ABL1IS >10% at six months after initiation of 2nd line TKI5
Sub-optimal Response on 2nd line TKI

Failure to achieve MMR can prevent patients from reaching their treatment goals7
Discontinued 2nd line TKI mainly due to resistance4
A patient with confirmed loss of major molecular response (MMR) one year after initiation of 2nd line TKI5
Intolerance on 2nd line TKI

Intolerance can prevent patients from meeting their goals8
Switched from their 2nd line TKI, due to intolerance within the first year of treatment4
A patient suffering from (moderate to severe) adverse events while on therapy or persistent (mild to moderate) adverse events, that is unresponsive to optimal management.5
These patients need to switch to a 3rd line treatment to be able to achieve their treatment goals by getting CML under control while preserving/improving QOL9
Choose SCEMBLIX with SUPERIOR efficacy and a FAVORABLE tolerability profile vs a 2nd-generation TKI in a Phase 3 trial.1

SCEMBLIX is a NOVEL STAMP inhibitor with a different mechanism of action specifically targeting the ABL myristoyl pocket2

More than Double the chance of achieving MMR than TKI's, as early as 12 weeks3 and durable to 96 weeks, higher percentage of patients achieving MR4 or Deeper1

2.7x more patients stayed on treatment vs TKI at week 961
CML, chronic myeloid leukemia; ABL 1, Abelson protein tyrosine kinase 1; TKI, Tyrosine Kinase Inhibitor; BCR, breakpoint cluster region; IS, International Scale; STAMP, specifically targeting the ABL1 myristoyl pocket; MMR, Major molecular response; MR4, BCR::ABL1IS 0.01%; QOL, Quality of life
The complete "SCEMBLIX" Approach
For Scemblix 20mg and 40mg Abbreviated Prescription Information
For Scemblix 20mg and 40mg Abbreviated Prescription Information
References
Hochhaus A, Réa D, Boquimpani C, Minami Y, Cortes JE, Hughes TP, Apperley JF, Lomaia E, Voloshin S, Turkina A, Kim DW. Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL. Leukemia. 2023 Mar;37(3):617-26.
Scemblix Egyptian Drug Authority (EDA) Approved leaflet 05/05/2025.
Food and Drug administration (FDA). Scemblix MULTI-DISCIPLINE REVIEW. available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/215358Orig1s000,Orig2s000MultidisciplineR.pdf . last accessed 16/6/2025
Chitanava T, Matvienko I, Shuvaev V, Voloshin S, Martynkevich I, Vlasova Y, Efremova E, Mileeva E, Pirkhalo A, Makarova T, Vlasik R. Long-term outcomes of third-line therapy with tyrosine kinase inhibitors in chronic phase chronic myeloid leukemia: A real-life experience. Frontiers in Oncology. 2023 Mar 16;13:1138683.
Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, Turkina A, Kim DW, Apperley JF, Abdo A, Fogliatto LM. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood, The Journal of the American Society of Hematology. SUPPLEMENT. 2021 Nov 25;138(21):2031-41
Hehlmann R, Cortes JE, Zyczynski T, GambacortiPasserini C, Goldberg SL, Mauro MJ, Michallet M, Simonsson B, Williams LA, Gajavelli S, DeGutis I. Tyrosine kinase inhibitor interruptions, discontinuations and switching in patients with chronicphase chronic myeloid leukemia in routine clinical practice: SIMPLICITY. American journal of
hematology. 2019 Jan;94(1):46-54National Comprehensive Cancer Network (NCCN). Guidelines Version 3.2025 Chronic Myeloid Leukemia. Available at:
https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf . Last accessed 16/7/2025Hochhaus A, Saussele S, Rosti G, Mahon FX, Janssen JJ, Hjorth-Hansen H, Richter J, Buske C, ESMO Guidelines Committee. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2017 Jul 1;28:iv41-51.
Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, Turkina A, Kim DW, Apperley JF, Abdo A, Fogliatto LM. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood, The Journal of the American Society of Hematology. 2021 Nov 25;138(21):2031-41.
Approved by Egyptian Drug Authority: HF0424OA4707/092025. Invalidation date: 18/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
HF0424OA4707/092025 |





