

Dosing and adminstration
Dosing options to accommodate your patients1
Image
| Patients previously treated with 2 TKIs:
There is also an option to take SCEMBLIX 40 mg tablets twice a day (AM + PM) |
Recommended dosage for patients who have the T315I mutation is 200 mg bid AM + PM
Image

| Avoid food consumption for at least 2 hours before and 1 hour after taking SCEMBLIX1 |
Image

| Swallow SCEMBLIX whole - do not break, crush, or chew them1 |
Image

| SCEMBLIX is available as film-coated tablets: 20 mg (6.2 mm diameter) and 40 mg (8.2 mm diameter)1 |
Based on pharmacokinetic parameters studied in an exposure-response analysis, the predicted
efficacy and safety profile of SCEMBLIX at the 80 mg qd dose is similar to that at the 40 mg bid dose.1
Missed Dose
Image
| Once-daily dosage regimen:
Twice-daily dosage regimens:
|
Dosage reductions
For the management of adverse reactions, reduce the SCEMBLIX dose as described in the table below1
Recommended dosage reductions for Scemblix for adverse reactions
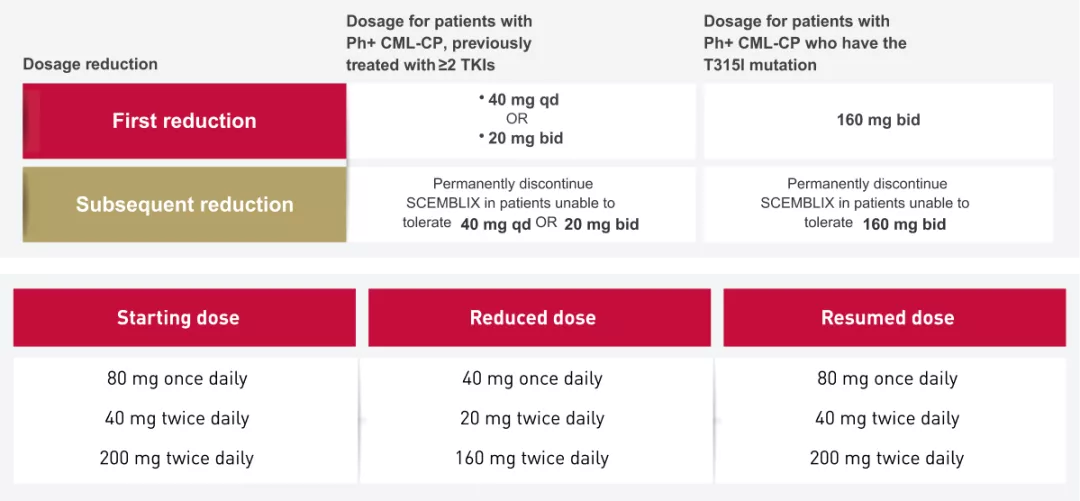
Dosage modifications for the management of adverse reactions1

Qd, once daily; Bid, twice daily; ANC: absolute neutrophil count; PLT: platelets; ULN: upper limit of normal; TKI, Tyrosine kinase inhibitor; PH+ CML-CP, Philadelphia chromosome–positive chronic myeloid leukemia chronic phase
For Scemblix 20mg and 40mg Abbreviated Prescription Information
For Scemblix 20mg and 40mg Abbreviated Prescription Information
References
Scemblix Egyptian Drug Authority (EDA) Approved leaflet 05/05/2025
Approved by Egyptian Drug Authority: HF0424OA4707/092025. Invalidation date: 18/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
HF0424OA4707/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
