
What is LEQVIO®?
LEQVIO® is indicated in adults with primary hypercholesterolemia (heterozygous familial and non-familial) or mixed dyslipidemia, as an adjunct to diet:
- in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin.
- alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated.
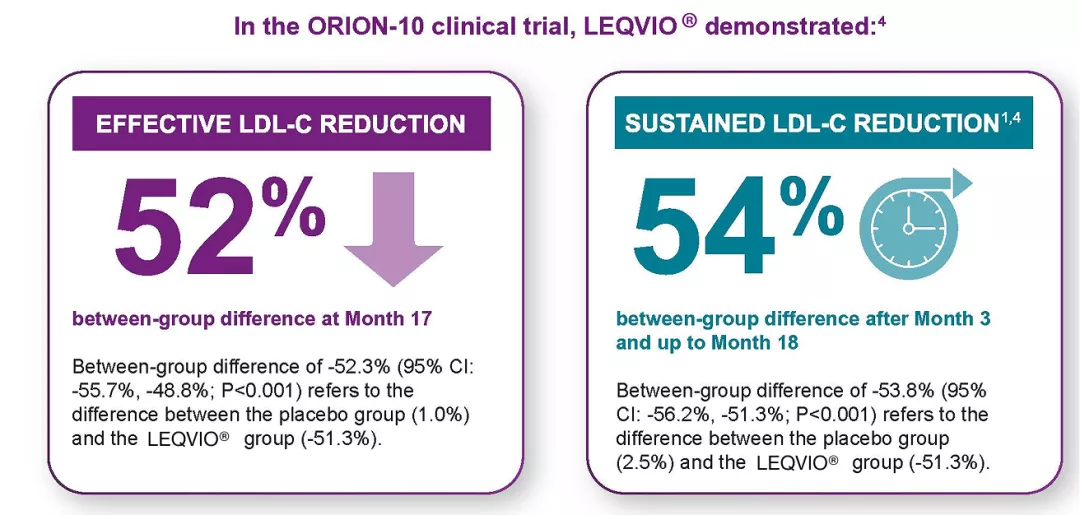
LEQVIO® is the first and only LDL-C-lowering therapy that is dosed twice a year. Reduction of LDL-C is expected to improve cardiovascular outcomes. (The effect of LEQVIO® on cardiovascular morbidity and mortality has not been determined.)1-4

*LEQVIO® is dosed initially, again at 3 months, and then once every 6 months.3
†LDL-C reduction was maintained during each 6-month dosing interval.3
Click Here to Download the Basic Succinct statement for LEQVIO®.
Choose LEQVIO® first for effective and sustained LDL-C reduction and as a strong complement to a maximally tolerated statin for your patients with ASCVD or HeFH 3-5
Abbreviations:
LDL-C, Low-density lipoprotein cholesterol; CI, Confidence interval; ASCVD, Atherosclerotic cardiovascular disease; HeFH, Heterozygous Familial Hypercholesterolemia.
LEQVIO® NSS - UAE
LEQVIO® NSS - UAE
References
LEQVIO® Product Summary. Novartis Europharm Limited. March 2024.
Wright RS, Raal FJ, Koenig W, et al. Inclisiran administration potently and durably lowers LDL-C over an extended-term follow-up: the ORION-8 trial. Cardiovasc Res. 2024 May 16:cvae109. doi: 10.1093/cvr/cvae109.
LEQVIO® Core Data Sheet. Novartis Europharm limited.
Ray KK, Wright RS, Kallend D, et al; ORION-10 and ORION-11 Investigators. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507-1519. doi:10.1056/NEJMoa1912387.
Raal FJ, Kallend D, Ray KK, et al; ORION-9 Investigators. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;1:1-11. doi:10.156/NEJMoa1913805.
https://www.novartis.com/news/media-releases/novartis-receives-eu-approval-leqvio-inclisiran-first-class-sirna-lower-cholesterol-two-doses-year





