
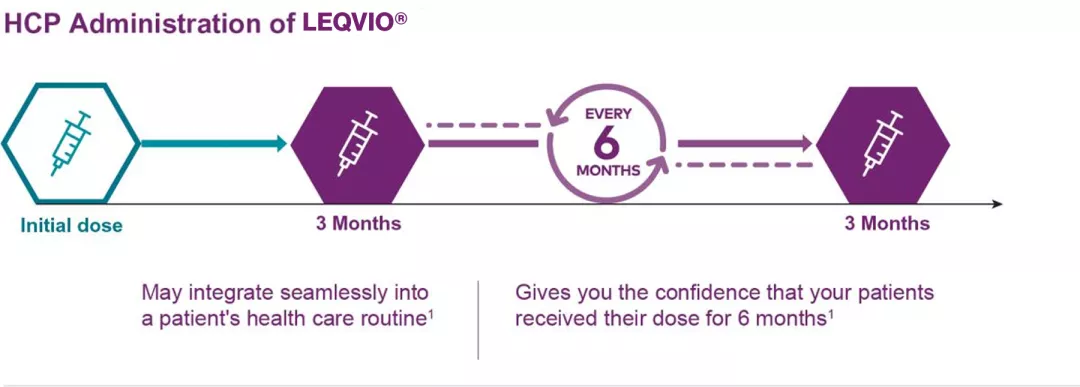
LEQVIO® Is the first and only LDL-C-lowering therapy that is dosed twice a year.1*
*LEQVIO® is dosed initially, again at 3 months, and then once every 6 months.1
†LEQVIO® should appear clear, pale yellow to colorless.1 This color variation is normal and has no impact on the overall quality of LEQVIO®.
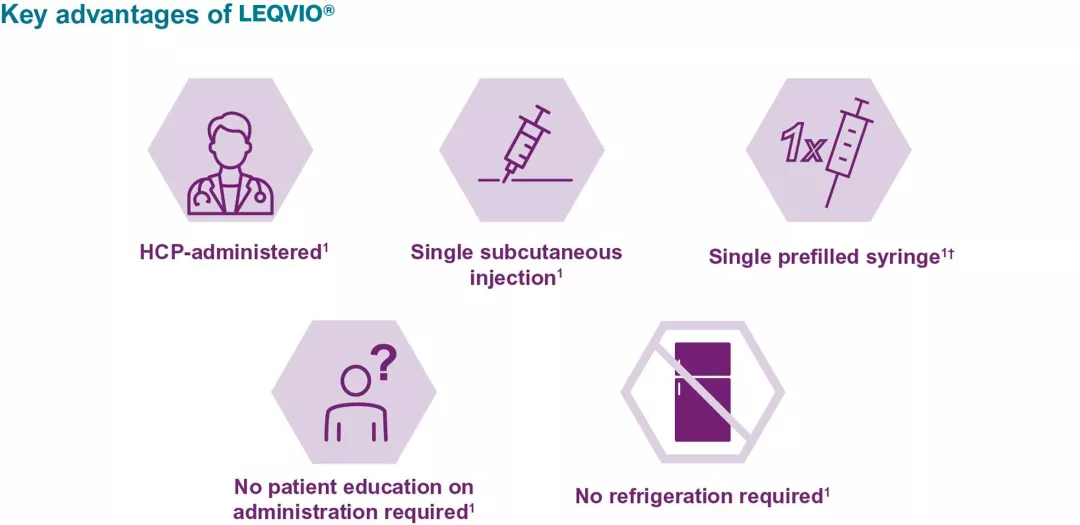
- Each prefilled syringe contains 284 mg LEQVIO® in 1.5 ml of solution1
- Do not store above 25 °C. Do not freeze2-6
- LEQVIO® is administered in the abdomen. Alternative injection sites include the upper arm or thigh1
Missed doses
- If a planned dose is missed by less than 3 months, LEQVIO® should be administered and dosing continued according to the patient's original schedule1
- If a planned dose is missed by more than 3 months, a new dosing schedule should be started-LEQVIO® should be administered initially, again at 3 months, followed by once every 6 months1
Special precautions for disposal1
LEQVIO® should be inspected visually prior to administration. The solution should appear clear, pale yellow to colorless. If the solution contains visible particulate matter, the solution should not be used.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
LEQVIO® NSS - UAE
LEQVIO® NSS - UAE
References
LEQVIO® . Core Data Sheet. Novartis Europharma limited.
Data on file. ORION-10 (MDCO-PCS-17-04) Clinical Study Report. The Medicines Company; 2019. "to be provided if requested."
Data on file. ORION-9 (MDC-PCS-17-03) Clinical Study Report. The Medicines Company; 2019. "to be provided if requested."
Data on file. ORION-11 (MDCO-PCS-17-08) Clinical Study Report. The Medicines Company; 2019."to be provided if requested."
Ray KK, Wright RS, Kallend D, et al; ORION-10 and ORION-11 Investigators. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020,382(16):1507-1519. doi:10.1056/NEJMoa1912387
Raal FJ, Kallend D, Ray KK, et al; ORION-9 Investigators. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engel K Med. 2020;1:1-11. doi:10.1056/NEJMoa1913805
