
V-INITIATE: LEQVIO® in a real-world setting
Study Design1
A 12-month, randomized, open-label, Phase IIIb study in patients with ASCVD (N=450) evaluated the efficacy and safety of early initiation with LEQVIO® immediately upon failure to reach LDL-C <70 mg/dL on maximally tolerated statin vs usual care in a real-world setting. A prospective, pragmatically designed trial, randomized patients 1:1 to inclisiran (284 mg at days 0, 90, and 270) plus usual care (lipid management at treating physician’s discretion) vs usual care alone.
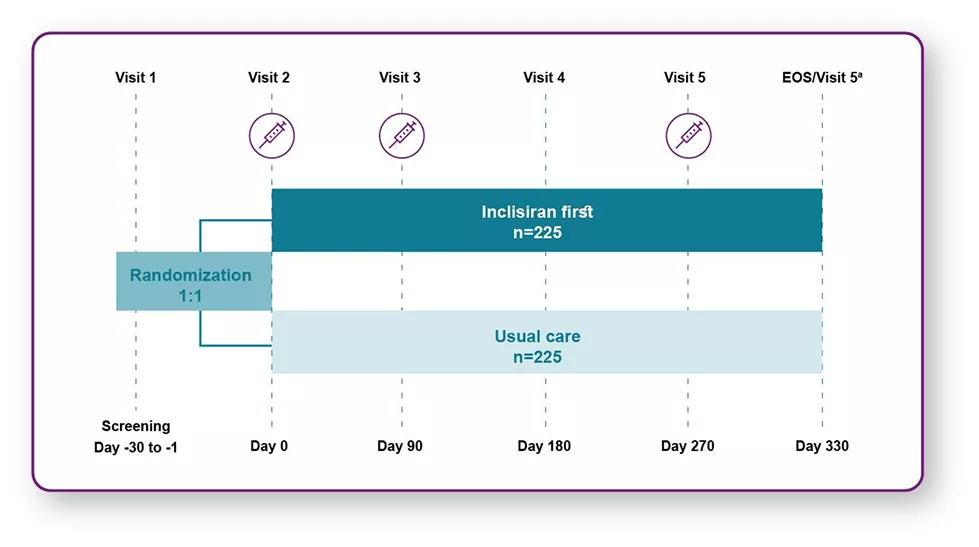
Patients with atherosclerotic cardiovascular disease with low-density lipoprotein cholesterol ≥70 mg/dL or non-high-density lipoprotein cholesterol ≥100 mg/dL, and fasting triglycerides <500 mg/dL, receiving maximally tolerated statin therapy (or with documented statin intolerance), were randomized 1:1 to receive inclisiran first or usual care. Syringes represent inclisiran doses. aPrimary analysis time point.
In the V-INITIATE clinical trial, LEQVIO® demonstrated:
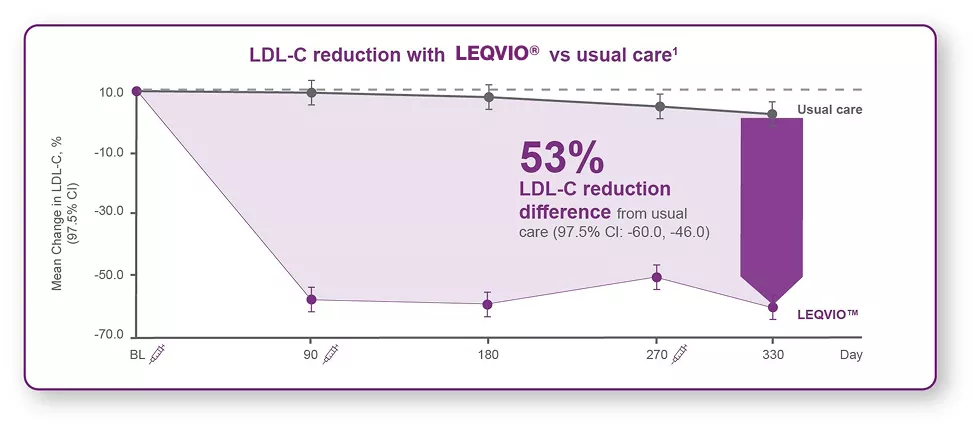
- 53% LDL-C reduction difference from usual care.1
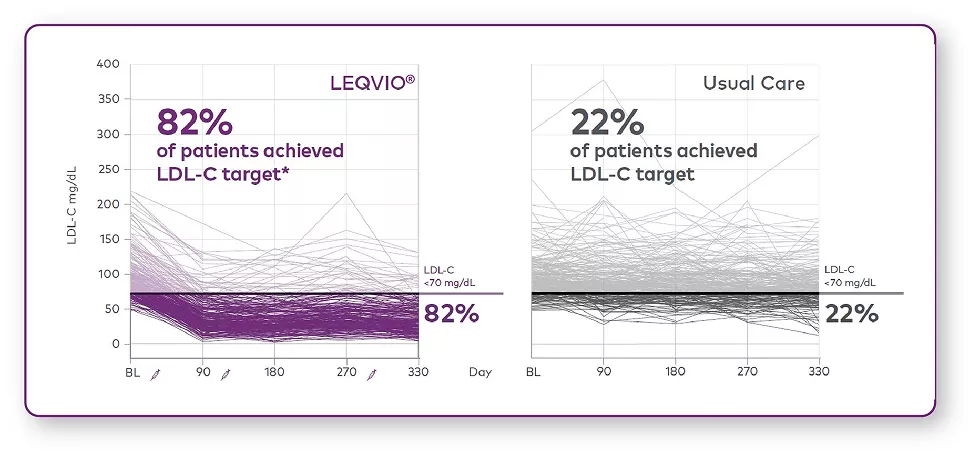
- 82% of patients with ASCVD achieved LDL-C target (<70 mg/dL) vs 22% in usual care.1
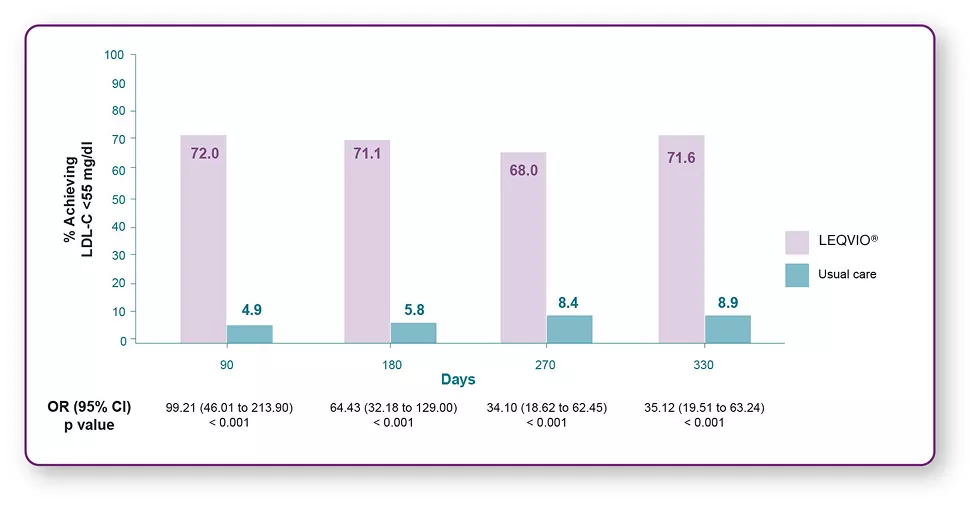
- 71.6% of patients with ASCVD achieved LDL-C target (<55 mg/dL) vs 8.9% in usual care.1
- The safety profile of LEQVIO® was consistent with pivotal trials.1,2
Primary endpoints: were percentage change in LDL-C from baseline and statin discontinuation rates.1
Early LEQVIO® use after failure to reach LDL-C target on statin1
V-INITIATE compared immediate LEQVIO® use after failure to reach LDL-C target with maximally tolerated statin vs usual care, which was physician-determined based on the 2018 AHA/ACC guidelines.1
Statin Discontinuation in LEQVIO® vs Usual Care1
V-INITIATE compared immediate LEQVIO® use after failure to reach LDL-C target with maximally tolerated statin vs usual care,which was physician-determined based on the 2018 AHA/ACC guidelines.1
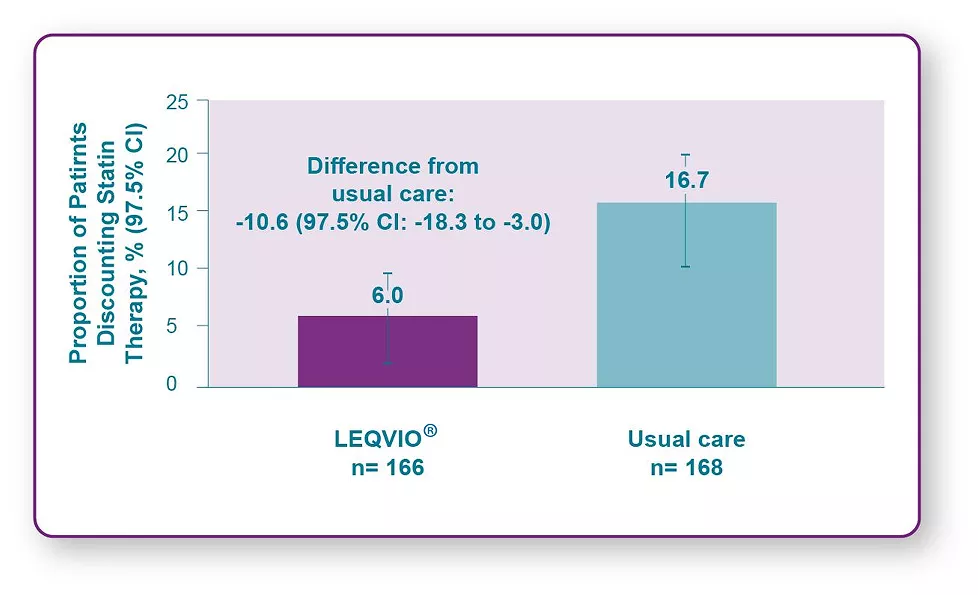
In the usual care arm, most patients (73%) remained on statins only, with a minority of patients receiving any additional non statin lipid-lowering therapy, including 4% of patients who received atl east 1 dose of LEQVIO®.1
Patients with ASCVD achieved LDL-C target (<70 mg/dL)1
Patients with ASCVD achieved LDL-C target (<55 mg/dL)1
Safety profile consistent with Phase III trials1,2
Injection site reactions were the most common cause for treatment discontinuation (0.2% of patients taking LEQVIO® vs 0% taking placebo)2
In V-INITIATE (N=450), a randomized open-label Phase IIIb study in a real-world setting:
- The safety profile of LEQVIO® was consistent with pivotal trials1,2
- No new safety signals1
ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; EOS, end of study; ACC, American College of Cardiology; AHA, the American Heart Association; CI, Confidence interval.
LEQVIO® NSS - UAE
LEQVIO® NSS - UAE
References
Koren MJ, Rodriguez F, East C, et al. An “Inclisiran First” Strategy vs Usual Care in Patients With Atherosclerotic Cardiovascular Disease. J Am Coll Cardiol. 2024 May 21;83(20):1939-1952.
LEQVIO®. Summary of product characteristics. Novartis Europe Limited.
