
LEQVIO® Demonstrated Effective and Sustained LDL-C Reduction1-3*
*LDL-C reduction was maintained during each 6-month dosing interval.1
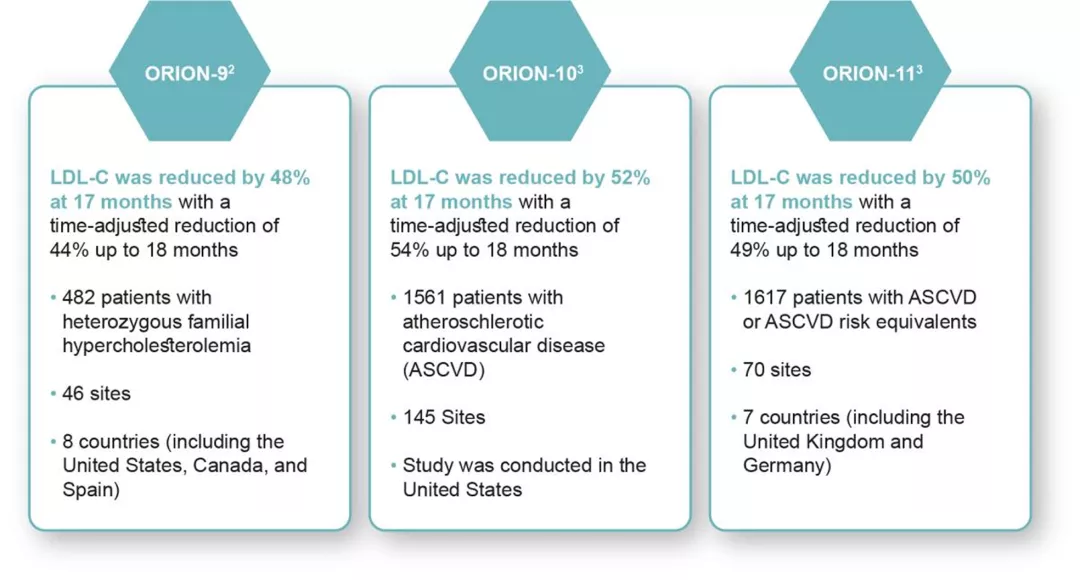
In 3 pivotal Phase III clinical trials across 18 months1
The ORION-9, -10, and -11 trials were multicenter, double-blind, randomized, placebo-controlled, 18-month studies. About 3600 patients were randomized to receive either placebo or LEQVIO® 284 mg treatment in addition to maximally tolerated statins with or without other lipid lowering therapy. Patients received an initial dose of LEQVIO® or placebo via subcutaneous injection, another at 3 months, and again every 6 months.1
Primary end points were met in all 3 trials:
- Percentage change in LDL-C from baseline to 17 months compared with placebo and time-adjusted
- Percentage change in LDL-C from baseline between 3 months and up to 18 months compared with placebo1
LEQVIO® NSS - UAE
LEQVIO® NSS - UAE
References
LEQVIO®. Core Data Sheet.Novartis Europharma limited.
Raal FJ, Kallend D, Ray KK, et al; ORION-9 Investigators. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engel K Med. 2020;1:1-11. doi:10.1056/NEJMoa1913805
Ray KK, Wright RS, Kallend D, et al; ORION-10 and ORION-11 Investigators. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020,382(16):1507-1519. doi:10.1056/NEJMoa1912387
