
LEQVIO® Was Well Tolerated1
In 3 pivotal Phase III clinical trials across 18 months1
- In clinical trials to date, >5000 patients were observed, with >5700 patient-years of LEQVIO® exposure2-6
- Most common adverse reactions were injection site reactions, which were predominantly mild (8.2% vs 1.8% placebo)1
- No clinically meaningful interactions are expected with LEQVIO®1
Most common adverse reactions in >3% of patients treated with LEQVIO® and more frequently than placebo2,3*
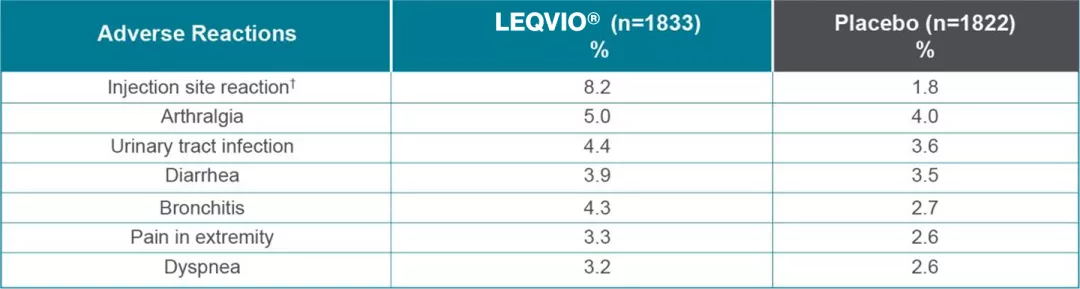
*Pooled analysis of ORION-9, ORION-10, and ORION-11 clinical trials.
†Includes related terms such as injection site pain, erythema, and rash.

Discontinuation due to adverse events was similar with patients taking LEQVIO® (2.5%) vs placebo (1.9%).2-3
Most common adverse events reported in one or more of the trials (>3% of patients treated with LEQVIO® and occurring more frequently than placebo)
were injection site reaction, arthralgia, urinary tract infection, diarrhea, bronchitis, pain in extremity, and dyspnea.2-3
The safety data are derived from 3 placebo-controlled trials (ORION-9, ORION-10, ORION-11).1
LEQVIO® NSS - UAE
LEQVIO® NSS - UAE
References
LEQVIO®. Core Data Sheet. Novartis Europharm limited.
Raal FJ, Kallend D, Ray KK, et al; ORION-9 Investigators. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;1:1-11. doi:10.1056/NEJMoa1913805.
Ray KK, Wright RS, Kallend D, et al; ORION-10 and ORION-11 Investigators. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020,382(16):1507-1519. doi:10.1056/NEJMoa19123879.
Data on file. Clinical Study Report - post hoc analysis supplemental table. Novartis Pharmaceuticals; 2019. "to be provided if requested"
Data on file. ORION-3 (MDCO-PCS-16-01) Safety Report. The Medicines Company; 2020. "to be provided if requested"
Data on file. ORION-8 (MDCO-PCS-17-05) Safety Report. The Medicines Company; 2020. "to be provided if requested"
