Pluvicto▼(lutetium [177Lu] vipivotide tetraxetan) prescribing information (external link)
Locametz▼(gozetotide) prescribing information (external link)


Mechanism of action (MoA)
Pluvicto®▼ (lutetium [177Lu] vipivotide tetraxetan) is indicated for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy or who are not medically suitable for taxanes.1
Pluvicto Summary of Product Characteristics (SmPC) can be found here.
Pluvicto is available in the United Kingdom to eligible private patients.
Locametz®▼ (gozetotide) is for diagnostic use only. Locametz, after radiolabelling with gallium-68, is a radioactive diagnostic agent indicated for the identification of prostate-specific membrane antigen (PSMA)-positive lesions by positron emission tomography (PET) in adult patients with prostate cancer.2
Pluvicto is a PSMA-targeted radioligand therapy (RLT) that delivers DNA-damaging radiation to PSMA-positive bone, nodal, and visceral metastases.1,3–7
Pluvicto targets PSMA-positive cells, including prostate cancer cells.1
The MoA of Pluvicto can be broken down into a few key steps:
Pluvicto comprises 2 key components:
lutetium-177, a cytotoxic radionuclide, and PSMA-617, a PSMA-targeting ligand.1
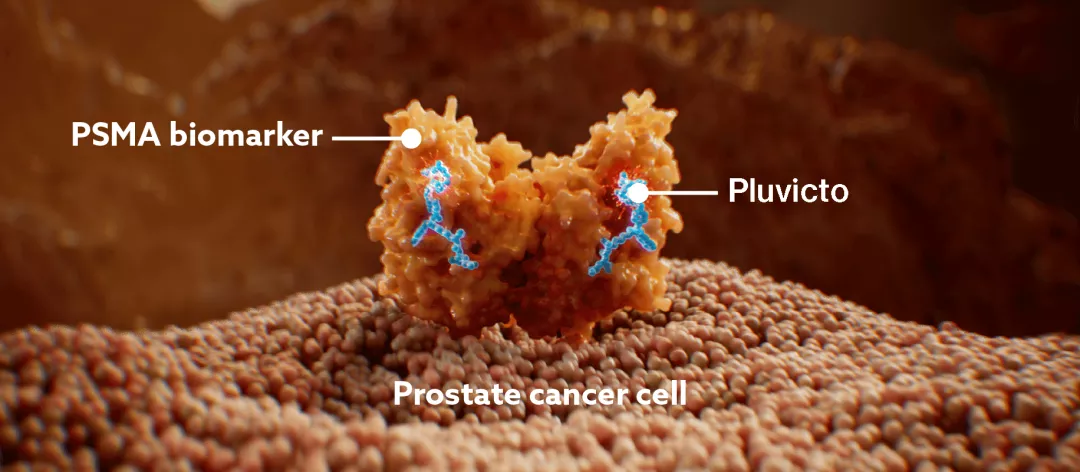
As determined by pre-clinical research, Pluvicto binds with high affinity to PSMA, a transmembrane protein overexpressed on prostate cancer cells.1
After binding to PSMA, Pluvicto undergoes endocytosis and is internalised into the cell.1,3
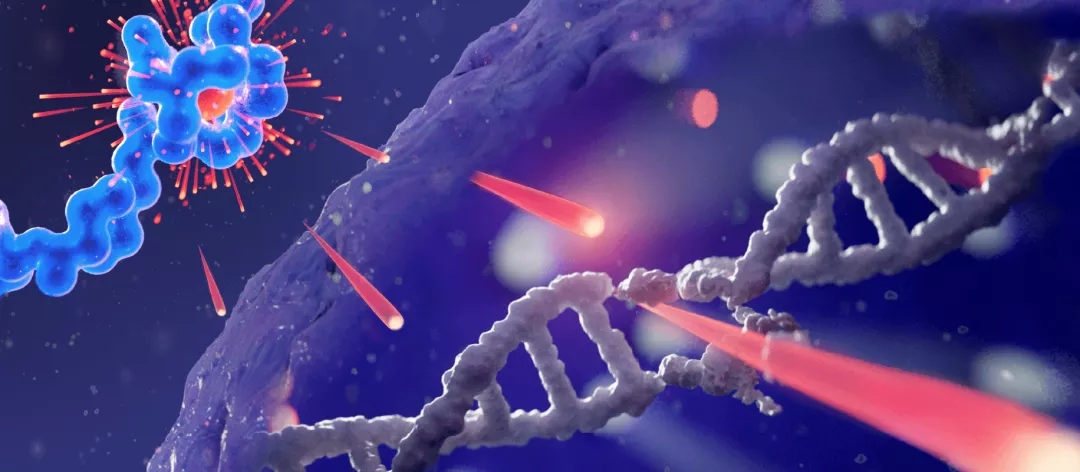
As determined by preclinical research, lutetium-177, (the cytotoxic radionuclide of Pluvicto), emits DNA-damaging radiation within the cell.1,3,5
The short path length of the radiation emitted by Pluvicto (approx. 2 mm max.), causes single- and double-stranded DNA breaks in targeted cells as well as surrounding cells, which can lead to cell death.4,5,10
PSMA scanning enables you to see and target PSMA-positive mCRPC2,5,11,12
The high sensitivity of PSMA scanning detects PSMA-positive bone, nodal or visceral metastases11–14
PSMA imaging establishes patient eligibility for PSMA-targeted RLT6
The most common adverse reactions (≥20%) occurring at a higher incidence in patients who received Pluvicto + BSoC include: fatigue (48.0%), dry mouth (39.3%), nausea (35.7%), anaemia (31.9%), decreased appetite (21.4%) and constipation (20.2%) .1 The most common grade 3 to 4 ADRs (≥5%) occurring at a higher incidence in patients who received Pluvicto + BSoC compared to BSoC alone include: anaemia (12.9%), thrombocytopenia (7.9%), lymphopenia (7.8%) and fatigue (6.6%).1
Please refer to the Pluvicto SmPC for full safety information.
177Lu, lutetium-177; ADR, adverse drug reaction; AR, androgen receptor; BSoC, best standard of care; DNA, deoxyribonucleic acid; mCRPC, metastatic castration-resistant prostate cancer; MoA, mechanism of action; PET, positron emission tomography; PSMA, prostate-specific membrane antigen; RLT, radioligand therapy; SmPC, summary of product characteristics.
References
Pluvicto®▼ (lutetium [177Lu] vipivotide tetraxetan) Summary of Product Characteristics.
Locametz®▼ (gozetotide) Summary of Product Characteristics.
Benešová M, et al. J Nucl Med 2015;56(6):914–920.
Fendler WP, et al. J Nucl Med 2017;58(11):1786–1792.
Hofman MS, et al. Lancet Oncol 2018;19(6):825–833.
Sartor O, et al. N Engl J Med 2021;385(12):1091–1103.
Violet J, et al. J Nucl Med 2019;60(4):517–523.
Liu H, et al. Cancer Res 1998;58(18):4055–4060.
Rajasekaran SA, et al. Mol Biol Cell 2003;14(12):4835–4845.
Ruigrok EAM, et al. Eur J Nucl Med Mol Imaging 2021;48(5):1339–1350.
Maffey-Steffan J, et al. Eur J Nucl Med Mol Imaging 2020;47(3):695–712.
Vlachostergios PJ, et al. Front Oncol 2021;11:630589.
Woythal N, et al. J Nucl Med 2018;59(2):238–243.
Schmuck S, et al. J Nucl Med 2017;58(12):1962–1968.
UK | December 2025 | FA-11470709-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.





