Pluvicto▼(lutetium [177Lu] vipivotide tetraxetan) prescribing information (external link)
Locametz▼(gozetotide) prescribing information (external link)


Clinical trials
Pluvicto®▼(lutetium [177Lu] vipivotide tetraxetan) is indicated for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy or who are not medically suitable for taxanes.1
Pluvicto Summary of Product Characteristics (SmPC) can be found here.
Pluvicto is available in the United Kingdom to eligible private patients.
Locametz®▼(gozetotide) is for diagnostic use only. Locametz, after radiolabelling with gallium-68, is a radioactive diagnostic agent indicated for the identification of PSMA-positive lesions by positron emission tomography (PET) in adult patients with prostate cancer.2
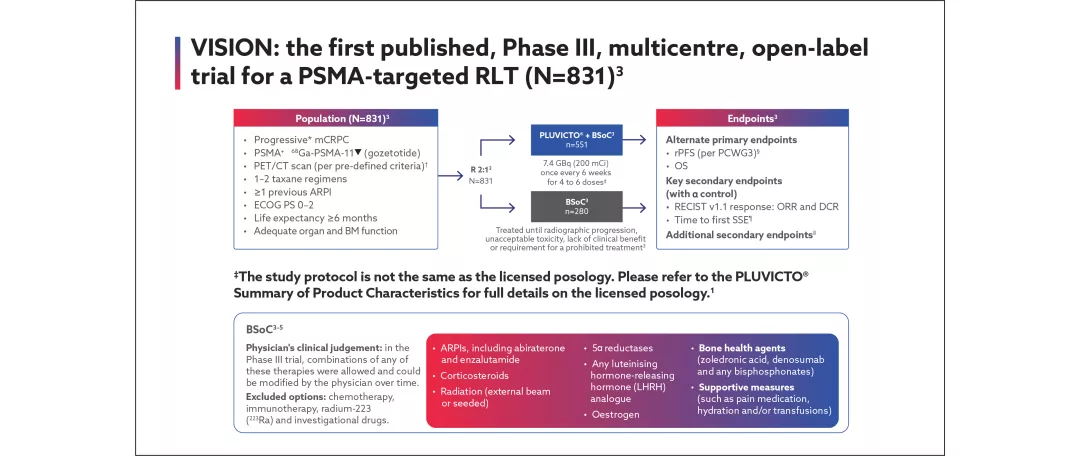
VISION
VISION was an international, prospective, randomised, open-label, multicentre, Phase III study (N=831) to assess the efficacy and safety profile of Pluvicto plus investigator-chosen best standard of care (BSoC) in the investigational arm, versus BSoC in the control arm. Patients with PSMA PET-scan positive mCRPC, and progression after prior taxane and androgen receptor (AR) pathway inhibitors, were randomised in a 2:1 ratio in favour of the investigational arm. The alternate primary endpoints were radiographic progression-free survival (rPFS) and overall survival (OS).3
View the study design for VISION below:


*Documented progressive mCRPC was based on at least one of three criteria: serum prostrate-specific antigen (PSA) progression, soft-tissue progression or bone disease progression.5 Soft tissue progression was defined as an increase of ≥20% in the sum of the diameter (SOD) of all target lesions based on the smallest SOD since treatment started or the appearance of one or more new lesions. Progression of bone disease was defined as evaluable disease or new bone lesion(s) by bone scan (2+2 Prostate Cancer Clinical Trials Working Group 3 [PCWG3] criteria). Serum PSA progression was defined as two consecutive increases in PSA over a previous reference value measured at least 1 week prior, minimal start value is 2.0 ng/mL.5
†PSMA-positive disease sites were defined as ≥1 PSMA-positive lesions anywhere in the body, with PSMA PET imaging ligand uptake greater than that of liver parenchyma in one or more metastatic lesions in any organ system. No size criteria were applied on PSMA-positive lesions.3
§Imaging-based progression-free survival (PFS) was defined as the time from randomisation to independently centrally-reviewed disease progression (defined according to the Prostate Cancer Clinical Trials Working Group 3 [PCWG3] criteria) or death.3
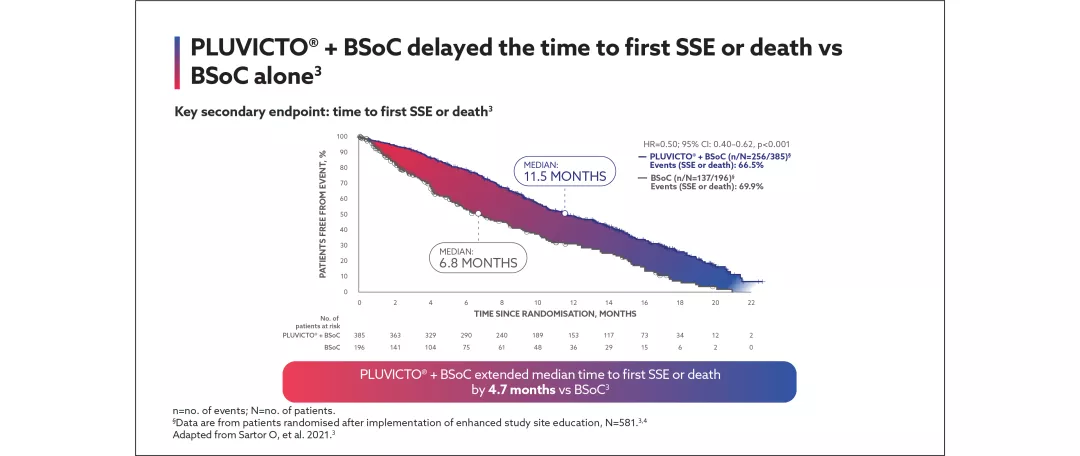
¶First symptomatic skeletal event (SSE) defined as first new symptomatic pathological bone fracture, spinal cord compression, tumour-related orthopaedic surgical intervention, requirement for radiation therapy to relieve bone pain or death from any cause, whichever occurred first.5
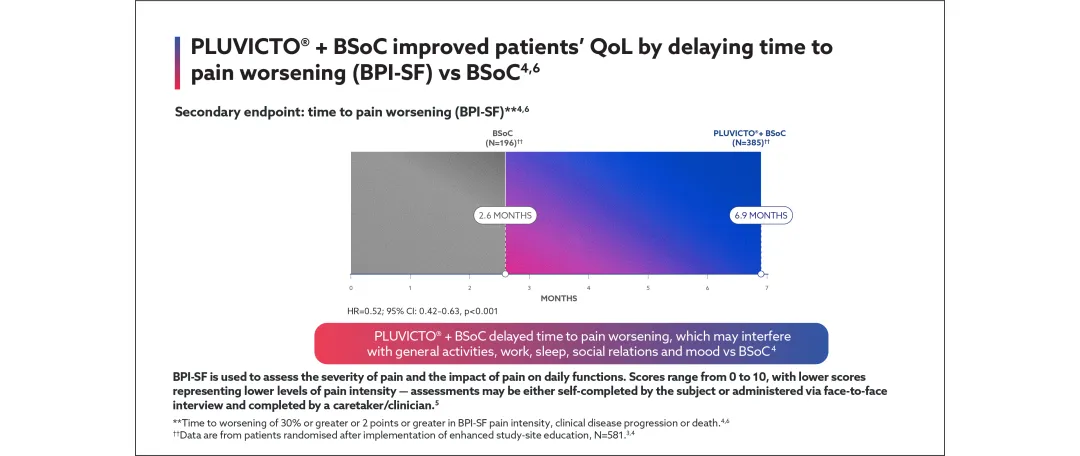
‖Additional secondary endpoints included: safety and tolerability; health-related quality of life (HRQoL): EQ-5D-5L, FACT-P and BPI-SF; health economics; composite PFS (radiological, clinical or PSA progression); biochemical response: PSA, alkaline phosphatase and lactic acid dehydrogenase levels.3
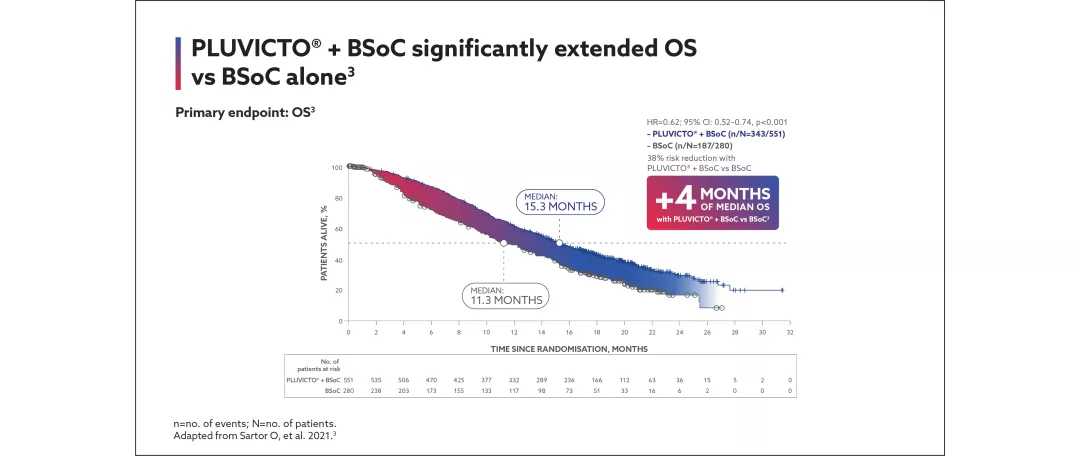
Pluvicto + BSoC significantly extends survival in PSMA-positive mCRPC patients vs BSoC alone3
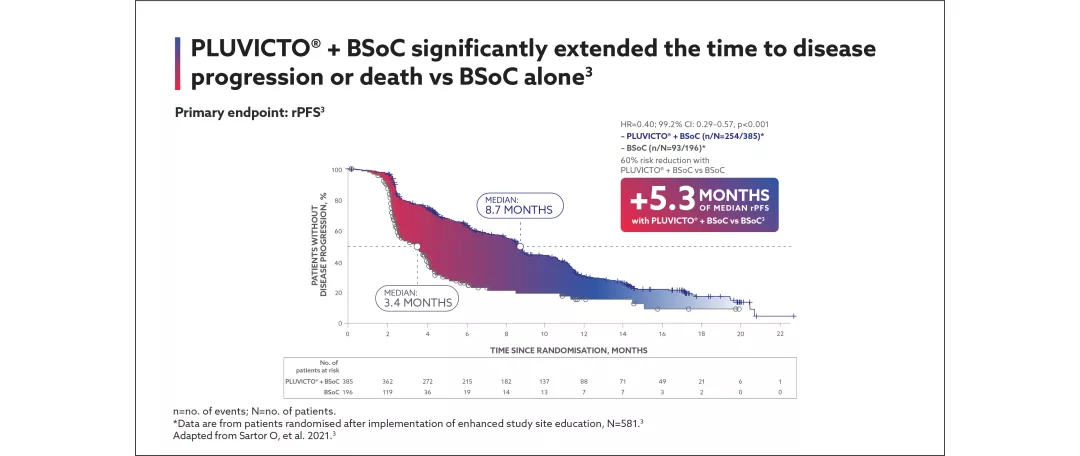
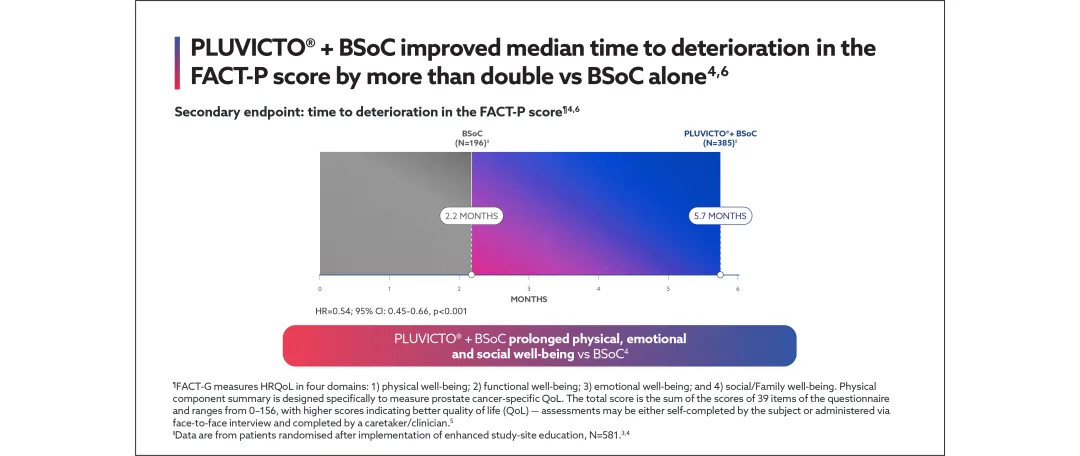
View the efficacy outcomes for VISION below:
Primary endpoints:


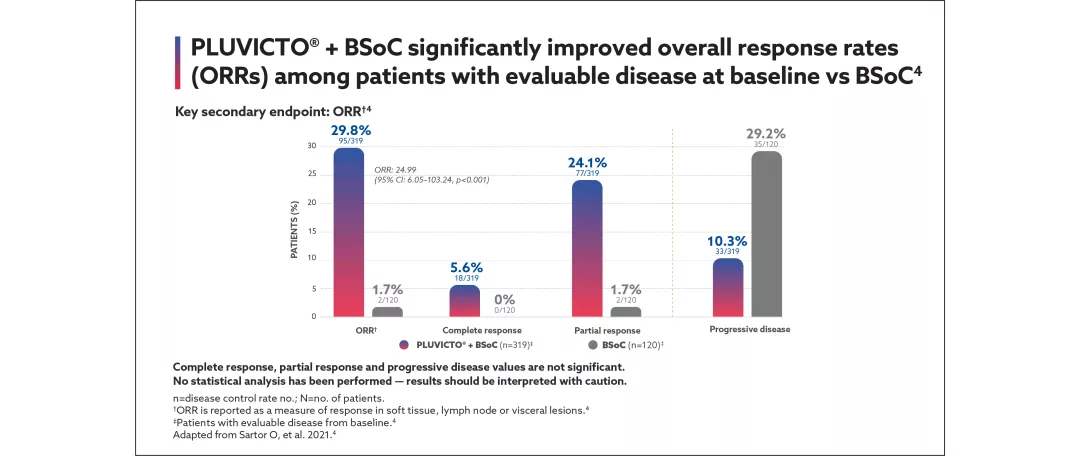
Secondary endpoints:




The most common adverse drug reactions (ADRs) (≥20%) occurring at a higher incidence in patients who received Pluvicto + BSoC compared to BSoC alone include: fatigue (43.1%), dry mouth (39.3%), nausea (35.3%), anaemia (31.8%), decreased appetite (21.2%) and constipation (20.2%). The most common grade 3 to 4 ADRs (≥5%) occurring at a higher incidence in patients who received Pluvicto + BSoC compared to BSoC alone include: anaemia (12.9%), thrombocytopenia (7.9%), lymphopenia (7.8%) and fatigue (6.6%).1
This list is not exhaustive; for further safety information please refer to the UK Summary of Product Characteristics.1
177Lu, lutetium-177; ADR, adverse drug reaction; AR, androgen receptor; ARPI, androgen receptor pathway inhibitor; BM, bone marrow; BPI-SF, brief pain inventory (short form); BSoC, best standard of care; CI, confidence interval; CNS, central nervous system; CT, computed tomography; DCR, disease control rate; ECOG-PS, Easter Cooperative Oncology Group performance status; EQ-5D-5L, 5-level EuroQol-5 dimension version; FACT-G, functional assessment of cancer therapy-general; FACT-P, functional assessment of cancer therapy-prostate; 68Ga-PSMA-II, gallium-68–labelled prostate-specific membrane antigen ligand II; HR, hazard ratio; HRQoL, health-related quality of life; LHRH, luteinising hormone releasing hormone; mCRPC, metastatic castration-resistant prostate cancer; MRI, magnetic resonance imaging; ORR, objective response rate; OS, overall survival; PCWG3, Prostate Cancer Clinical Trials Working Group 3; PET, positron emission tomography; PFS, progression-free survival; PSA, prostrate-specific antigen; PSMA, prostate-specific membrane antigen; QoL, quality of life; 223Ra, radium-223; R, randomisation; RECIST, Response Evaluation Criteria in Solid Tumors; RLT, radioligand therapy; rPFS, radiographic progression-free survival; SmPC, summary of product characteristics; SOD, sum of diameter; SSE, symptomatic skeletal event.
References
Pluvicto®▼ (lutetium [177Lu] vipivotide tetraxetan) Summary of Product Characteristics.
Locametz®▼ (gozetotide) Summary of Product Characteristics.
Sartor O, et al. N Engl J Med 2021;385(12):1091–1103.
Sartor O, et al. N Engl J Med 2021;385(12):1091–1103. Supplementary appendix.
Sartor O, et al. N Engl J Med 2021;385(12):1091–1103. Study protocol.
Fizazi K, et al. Ann Oncol 2021;32 (Supplement 5):S626–S677.
UK | January 2026 | FA-11470195-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.