

Dosing and administration of TAFINLAR® (dabrafenib) + MEKINIST® (trametinib)
TAFINLAR in combination with MEKINIST is indicated in adult patients with advanced non-small cell lung cancer (NSCLC) with a BRAF V600 mutation.1,2
For the full safety profile, please refer to the Summary of Product Characteristics (SmPC) for TAFINLAR and MEKINIST.
Adverse event reporting: Details of how to report adverse events are available at the bottom of the page. Please refer to the respective SmPC for all licensed indications.
TAFINLAR + MEKINIST offers oral dosing1–4
The key information on dosing and administration of TAFINLAR + MEKINIST provided below may be helpful when discussing treatment management with your patients.
| TAFINLAR | MEKINIST |
If a patient forgets to take TAFINLAR or MEKINIST | If the missed dose is less than 6 hours late, the patient must take it as soon as they remember. If the missed dose is more than 6 hours late, the patient should skip that dose and take the next dose at the usual time. Then carry on taking the capsules at regular times as usual. The patient must not take a double dose to make up for a missed dose. | If the missed dose is less than 12 hours late, the patient must take it as soon as they remember. If the missed dose is more than 12 hours late, the patient should skip that dose and take the next dose at the usual time. Then carry on taking the capsules at regular times as usual. The patient must not take a double dose to make up for a missed dose. |
If a patient vomits after taking TAFINLAR or MEKINIST | The patient must not take the capsules or tablet again but wait until next dose is due and take it at the normal time.1,2 | |
If a patient has taken more than the prescribed number of TAFINLAR capsules, MEKINIST tablets, or other medicines | The patient must consult a doctor, pharmacist or nurse immediately, and take the medicine(s) and its packaging with them if seeing them face to face. | |
The management of adverse events (AEs) associated with TAFINLAR + MEKINIST treatment may require dose reduction, treatment interruption or treatment discontinuation.1,2
Dose modification schedule based on the grade of any AEs1,2
Pyrexia
If a patient’s temperature is ≥38 °C therapy should be interrupted. Treatment with antipyretics should be initiated. The use of oral corticosteroids should be considered in those instances in which antipyretics are insufficient.
If the patient is symptom free for at least 24 hours, therapy should be restarted, either:
At the same dose level, or
Reduced by one dose level if the pyrexia is recurrent and/or was accompanied by other severe symptoms, including dehydration, hypotension or renal failure
Grade (CTC-AE)* | Recommended TAFINLAR dose modification | Recommended MEKINIST dose modification |
Grade 1 or Grade 2 (tolerable) | Continue treatment and monitor as clinically indicated | Continue treatment and monitor as clinically indicated |
Grade 2 (intolerable) or Grade 3 | Interrupt therapy until toxicity is Grade 0–1 and reduce by one dose level when resuming therapy | Interrupt therapy until toxicity is Grade 0–1 and reduce by one dose level when resuming therapy |
Grade 4 | Discontinue permanently, or interrupt therapy until Grade 0–1 and reduce by one dose level when resuming therapy | Discontinue permanently, or interrupt therapy until Grade 0–1 and reduce by one dose level when resuming therapy |
*The intensity of clinical adverse events graded by the Common Terminology Criteria for Adverse Events v4.0 (CTC-AE): Grade 1: Mild; intervention not indicated. Grade 2: Moderate; minimal, local or non-invasive intervention indicated. Grade 3: Severe or medically significant but not immediately life-threatening; hospitalisation or prolongation of hospitalisation indicated. Grade 4: Life-threatening consequences; urgent intervention indicated.1,2
Dose level | TAFINLAR dose | MEKINIST dose |
Starting dose | 150 mg twice daily | 2 mg once daily |
1st dose reduction | 100 mg twice daily | 1.5 mg once daily |
2nd dose reduction | 75 mg twice daily | 1 mg once daily |
3rd dose reduction | 50 mg twice daily | 1 mg once daily |
| Dose adjustment of TAFINLAR <50 mg twice daily is not recommended | Dose adjustment of MEKINIST <1 mg once daily is not recommended |
Recommended dose level reductions
TAFINLAR and MEKINIST should be simultaneously dose reduced, interrupted or discontinued. Exceptions, where dose modifications are necessary for only one of the two treatments, are shown in the table below.3,4
Cases where dose modifications are necessary for only one of the two treatments1,2
Please refer to the SmPCs and Patient Information Leaflets (PILs) for advice on administration, dose modifications, storage and handling of TAFINLAR and MEKINIST.
Modification of TAFINLAR dose only | Modification of MEKINIST dose only |
|
|
See the TAFINLAR and MEKINIST SmPCs for further information on dose adjustments.
Recommended dose modifications for MEKINIST for RPED1,2
Grade 1 | Continue treatment with retinal evaluation monthly until resolution. If RPED worsens follow instructions below and withhold MEKINIST for up to 3 weeks. |
Grade 2–3 | Withhold MEKINIST for up to 3 weeks. |
Grade 2–3 (improves to Grade 0–1 within 3 weeks) | Resume MEKINIST at a lower dose (reduced by 0.5 mg) or discontinue trametinib in patients taking trametinib 1 mg daily. |
Grade 2–3 (does not improve to Grade 0–1 within 3 weeks) | Permanently discontinue MEKINIST. |

Children and adolescents (<18 years)
The safety and efficacy of TAFINLAR + MEKINIST have not yet been established in children and adolescents (<18 years)
Elderly
No adjustment of the initial dose of TAFINLAR or MEKINIST is required in patients >65 years of age
More frequent dose adjustments of MEKINIST may be required in patients >65 years of age
Renal impairment
Mild to moderate: No dose adjustment of TAFINLAR or MEKINIST is required
Severe: Use with caution when administered as monotherapy or in combination therapy. There are no clinical data for TAFINLAR or MEKINIST in patients with severe renal impairment and the potential need for dose adjustment cannot be determined

Hepatic impairment
Mild: No dose adjustment of TAFINLAR or MEKINIST is required
Moderate to severe: TAFINLAR + MEKINIST should be used with caution in patients with moderate or severe hepatic impairment when administered as monotherapy or in combination
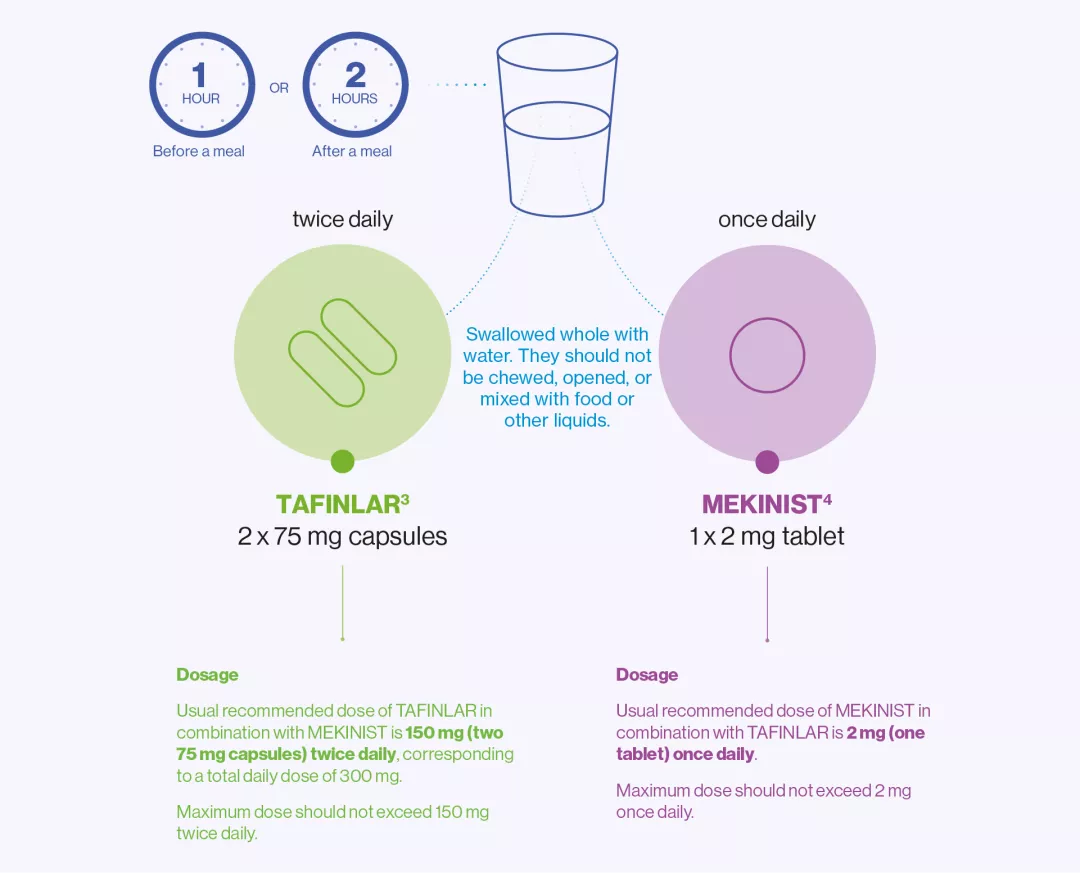
Administration of TAFINLAR + MEKINIST
The diagram below provides an overview on how to take TAFINLAR + MEKINIST.3,4
Please do not share this page directly with patients.
Always encourage your patients to carefully read the PILs in their TAFINLAR and MEKINIST packs before starting treatment, as they contain important information for them.
| TAFINLAR | MEKINIST |
How much to take | The usual dose is 150 mg (two 75 mg capsules) twice daily. | The usual dose is 2 mg (one tablet) once a day. |
When to take | TAFINLAR should be taken twice a day, in the morning and in the evening, about 12 hours apart. The morning and evening doses of TAFINLAR should be taken at similar times every day. | MEKINIST should be taken once a day, at about the same time each day, either with the morning or with the evening dose of TAFINLAR. |
How to take | TAFINLAR capsules should be swallowed whole with water. They should not be chewed or opened and should not be mixed with food or liquids due to chemical instability of dabrafenib. | TAFINLAR capsules should be swallowed whole with water. They should not be chewed or opened and should not be mixed with food or liquids due to chemical instability of TAFINLAR. |
AE, adverse event; BRAF V600, mutation of the BRAF gene at valine (V) 600; CTC-AE, Common Terminology Criteria for Adverse Events; ILD, interstitial lung disease; LLN, lower limit of normal; LVEF, left ventricular ejection fraction; NSCLC, non-small cell lung cancer; PIL, patient information leaflet; RAS, rat sarcoma; RPED, retinal pigment epithelial detachment; RVO, retinal vein occlusion; SmPC, summary of product characteristics.
References
TAFINLAR (dabrafenib) Summary of Product Characteristics.
MEKINIST (trametinib) Summary of Product Characteristics.
TAFINLAR (dabrafenib) Patient Information Leaflet.
MEKINIST (trametinib) Patient Information Leaflet.
UK | March 2025 | FA-11220646
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.


