

BRAF mutation and mechanism of action for TAFINLAR® (dabrafenib) + MEKINIST® (trametinib)
TAFINLAR in combination with MEKINIST is indicated in adult patients with advanced non-small cell lung cancer (NSCLC) with a BRAF V600 mutation.1,2
For the full safety profile, please refer to the Summary of Product Characteristics (SmPC) for TAFINLAR and MEKINIST.
Adverse event reporting: Details of how to report adverse events are available at the bottom of the page. Please refer to the respective SmPC for all licensed indications.
BRAF represents a novel therapeutic target for the treatment of advanced NSCLC3
Testing for BRAF in the UK
In England, BRAF testing can now be done as part of the next-generation sequencing (NGS) panel. For more information, go to the national genomic test directory for cancer in England.4
Molecular characterisation of NSCLC marked a turning point in the treatment of lung tumours harbouring kinase alterations suitable for targeted drug inhibition.3
The role of BRAF mutations in NSCLC
Approximately 1.5–3.5% of patients with NSCLC have a BRAF mutation.3
BRAF mutations lead to constitutive activation of the mitogen-activated protein kinase (MAPK) pathway.5
BRAF V600E is the most common variant, occurring in roughly 50% of BRAF-mutated tumours.6
NSCLC with BRAF V600E mutations has histological features suggestive of an aggressive tumour.3
In patients with NSCLC who received platinum-based chemotherapy, outcomes were less favourable among those with a BRAF V600E-mutated tumour vs those with BRAF wild-type (WT) tumours.5
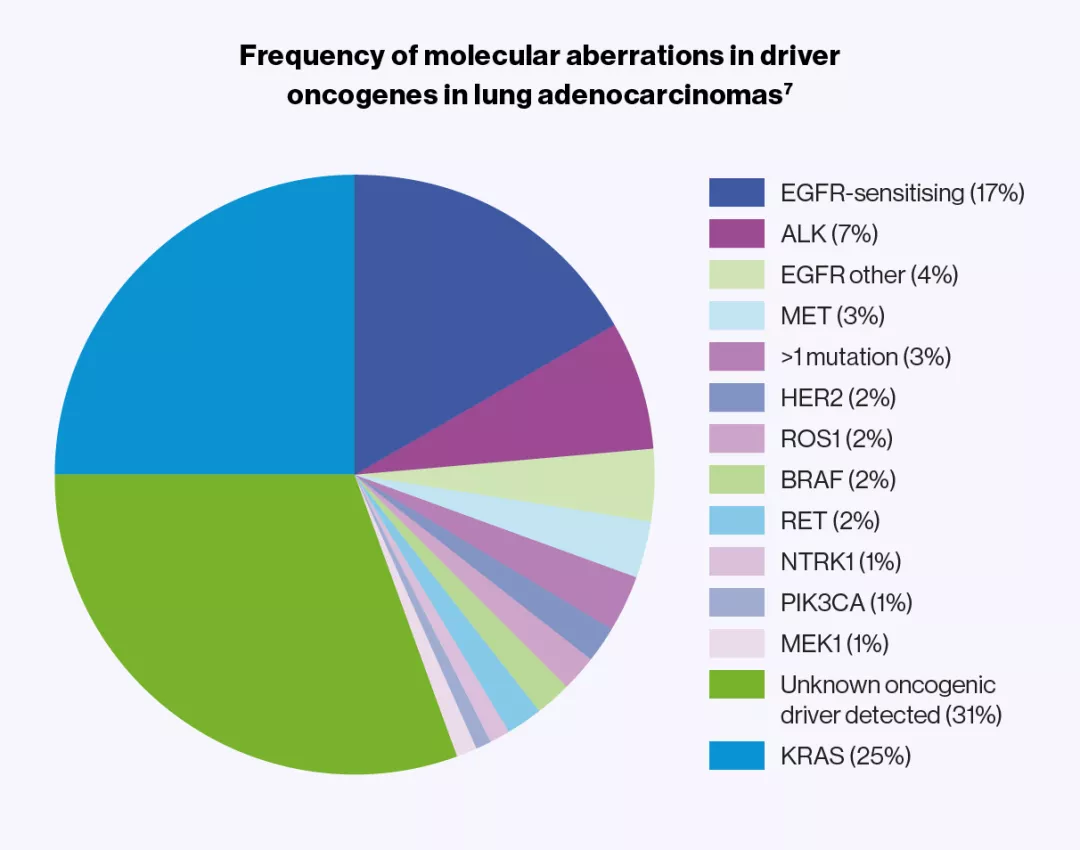
Adapted from Hirsch FR, et al. 2017.7
TAFINLAR + MEKINIST target two distinct points on the MAPK pathway to provide concomitant inhibition1,2
Mechanism of action for TAFINLAR + MEKINIST
TAFINLAR + MEKINIST target two different kinases in the MAPK pathway (BRAF and MEK1/2, respectively) to block the signalling that leads to abnormal cell division and growth.1,2
Combination therapy with TAFINLAR + MEKINIST results in prolonged inhibition of tumour growth compared with either drug alone in BRAF V600E-mutant tumours, both in vitro and in vivo.1,2
Schematic of the MAPK signalling pathway showing where TAFINLAR and MEKINIST act3
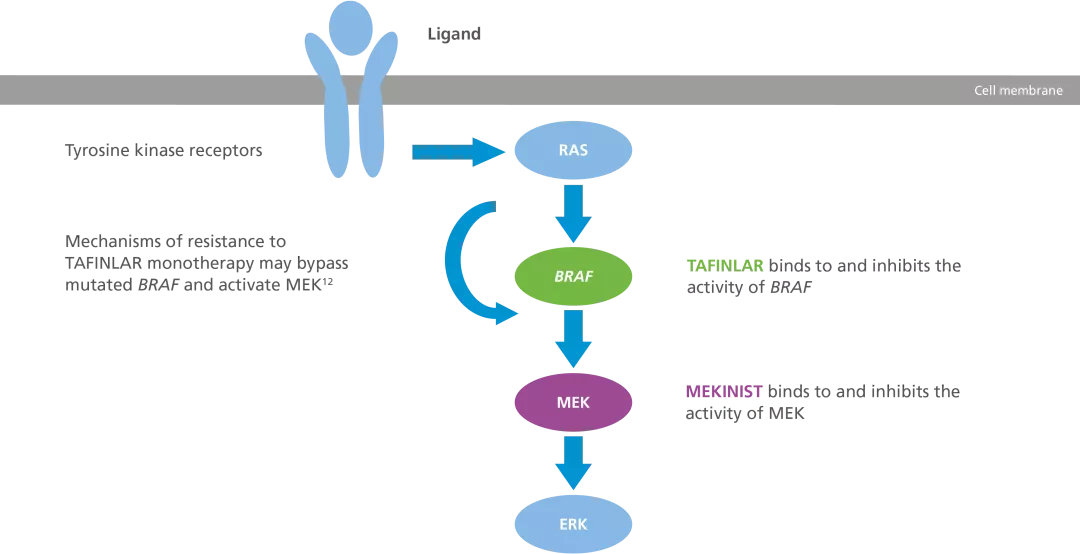
Adapted from Leonetti A, et al. 2018.3
ALK, anaplastic lymphoma kinase; BRAF, v-raf murine sarcoma viral oncogene homolog B1; BRAF V600, mutation of the BRAF gene at valine (V) 600; BRAF V600E, mutation of the BRAF gene at valine (V) 600 to glutamate (E); EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinases; HER2, human epidermal growth factor receptor-2; KRAS, Kirsten rat sarcoma viral oncogene homolog; MAPK, mitogen-activated protein kinase; MEK, mekinist; MEK1/2, mitogen-activated protein kinase 1/2; MET, mesenchymal epithelial transition factor receptor; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; NTRK1, neurotrophic receptor tyrosine kinase 1; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; RAS, rat sarcoma; RET, ret proto-oncogene; ROS1, proto-oncogene 1, receptor tyrosine kinase; SmPC, summary of product characteristics; WT, wild-type.
References
TAFINLAR (dabrafenib) Summary of Product Characteristics.
MEKINIST (trametinib) Summary of Product Characteristics.
Leonetti A, et al. Cancer Treat Rev 2018;66:82–94.
NHS England. National Genomic Test Directory for cancer. Available at: https://www.england.nhs.uk/publication/national-genomic-test-directories/ [Accessed April 2025].
Planchard D, et al. Lancet Oncol 2016;17:984–993.
O'Leary C, et al. Transl Lung Cancer Res 2019;8:1119–1124.
Hirsch FR, et al. Lancet 2017;389:299–311.
UK | April 2025 | FA-11392355
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.


