

ENTRESTO UK real-world evidence
ENTRESTO is indicated in adult patients for the treatment of symptomatic chronic heart failure with reduced ejection fraction.1
For full safety information, please refer to the Summary of Product Characteristics.1
Please see the below examples of ENTRESTO use in the real-world setting.
Please see the SmPC for further information and initial and following dose adjustments depending on certain conditions.
1. South of England – Retrospective comparison of 552 patients over 2 years2
This study was published in Heart, an international peer-reviewed journal from BMJ and BCS publishing. No conflicts of interest were reported.
Objective of the study
Investigate the extent to which the real-world use of ENTRESTO mirrors that of the PARADIGM-HF population and complies with guideline recommendations.
Overview
Study | Background | Objective | Findings |
Real-world use of ENTRESTO in the South of England
| Design: Retrospective comparison of n=552 patients started on ENTRESTO between 2017 and 2019 at 4 centres in the South of England with those from the ENTRESTO arm of the PARADIGM-HF study. In the final analysis, n=438 patients were included. | Investigate the extent to which the real-world use of ENTRESTO mirrors that of the PARADIGM-HF population and complies with guideline recommendations. |
|
Key findings
Cardiologists from the 4 South of England centres studied have prescribed ENTRESTO in a wide range of eligible HF patients in line with the licensed indication.
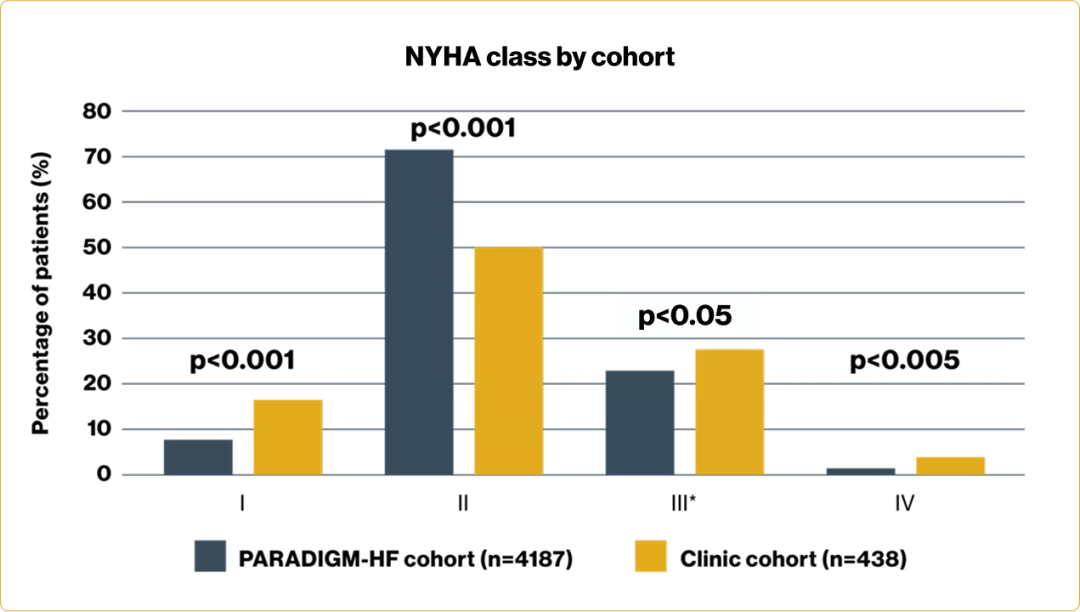
Adapted from Di Marco A, et al. 2020.2
Baseline characteristics of the South of England population
Baseline characteristics2 | |||
PARADIGM-HF cohort N=4187 | Clinic patients N=438 | p value | |
Age (years) | 63.8 ± 11.5 | 68.7 ± 12 | <0.001 |
Female sex | 879 (21.0) | 71 (20.3) | 0.01 |
SBP mmHg | 122 ± 15 | 123 ± 20 | 0.18 |
Heart rate – bpm | 72 ± 12 | 69 ± 12 | <0.001 |
Creatinine – mg/dl | 1.13 ± 0.3 | 1.13 ± 0.3 | 1.0 |
Clinical features | |||
Ischaemic cardiomyopathy | 2506 (59.9) | 219 (50.0) | <0.001 |
LVEF – % | 29.6 ± 6.1 | 29.9 ± 4.8 | 0.69 |
Medical history | |||
Hypertension | 2969 (70.9) | 156 (35.6) | <0.001 |
Diabetes | 1451 (34.7) | 112 (25.6) | <0.001 |
Numbers are total (%) or mean ± standard deviation | |||
92.5% of clinic patients had no exclusion criteria
4 had an eGFR <30 mL/min/1.73 m2 at baseline*
30 had an SBP <100 mmHg†
74% of clinic patients fulfilled inclusion criteria
68 were NYHA class I; 47 had an LVEF ≥35%
*ENTRESTO is not recommended in patients with end-stage renal disease, and concomitant use with aliskiren‐containing medicinal products in patients with diabetes mellitus or in patients with renal impairment (eGFR <60 ml/min/1.73 m2) is contraindicated. Use with caution in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2). (See section 5.1 of the Summary of Product Characteristics). Starting dose of 24 mg/26 mg twice daily and slow dose titration (doubling every 3–4 weeks) are recommended in these patients1
†Treatment should not be initiated unless SBP is ≥100 mmHg. Patients with SBP <100 mmHg were not studied.1
Outcomes | |||
PARADIGM-HF cohort N=4187 | Clinic patients N=438 | p value | |
ENTRESTO discontinued | 746 (17.8) | 61 (13.9) | 0.04 |
Dose of ENTRESTO at last assessment | 375 ± 71 mg | 297 ± 120* | <0.001 |
Decline in renal function (50% or more in eGFR)‡ | 94 (2.2) | 18 (6.5)† | <0.001 |
New offset AF | 84 (2.0) | 7 (1.6) | 0.55 |
Elevated serum creatinine ≥2.5 mg/dl | 139 (3.3) | 12 (2.7) | 0.51 |
Elevated serum creatinine ≥3.0 mg/dl | 63 (1.5) | 12 (2.7) | 0.05 |
New onset cough | 474 (11.3) | 10 (2.3) | <0.001 |
Angiodema | 19 (0.4) | 2 (0.5) | 0.99 |
Symptomatic hypotension with SBP <90§ | 112 (2.7) | 7 (1.6) | 0.13 |
Numbers are total (%) or mean ± standard deviation | |||
*Excludes patients where ENTRESTO was stopped.
†275 results only.
‡Use of sacubitril/valsartan may be associated with decreased renal function. The risk may be further increased by dehydration or concomitant use of NSAIDs. Down-titration should be considered in patients who develop a clinically significant decrease in renal function.1
§If hypotension occurs during treatment, temporary down-titration or discontinuation of sacubitril/valsartan is recommended.1
Conclusions
Baseline characteristics of the clinic population were broadly similar to those treated in the PARADIGM-HF study; ENTRESTO was prescribed in a wide range of HF patients and in some aspects was better tolerated than the trial data suggested.
2. Northern Ireland – NYHA class post-treatment with ENTRESTO6
Novartis sponsored Br J Cardiol 2019;26(suppl 1): peer-reviewed journal. The sponsored supplement was initiated and funded by Novartis Pharmaceuticals UK Ltd. Editorial control was retained by the authors and editors but Novartis reviewed the supplement for technical accuracy and compliance with relevant regulatory requirements before publication.
Objective of the study
Review of how ENTRESTO has been initiated by HF nurse specialists in Northern Ireland, the management of its side effects and its effect on patient outcomes.
Overview
Study | Background | Objective | Findings |
Real-world experience and clinical data with ENTRESTO: a Northern Ireland perspective:
| The Southern Health and Social Care Trust in Northern Ireland has a nurse-led HF service, with 7 HF nurse specialists serving approximately 1500 patients. HF patients can be reviewed in either a domiciliary, clinic or acute setting, with nurses supported by consultant cardiologists, renal consultants, GPs and a cardiology pharmacist. The nurses also have access to cardiac investigations. HF nurse specialists in the Trust began to initiate patients on ENTRESTO in September 2016 following publication of the NICE guidance. | To review how ENTRESTO has been initiated by HF nurse specialists in Northern Ireland, the management of its side effects and its effect on patient outcomes. |
|
Key findings
Prescribing ENTRESTO
Patients were prescribed ENTRESTO if they met the following criteria from the NICE guidance:
- NYHA class II or above
- LVEF ≤35%
- Already established on an ACEi or ARBNo extra clinics were run, and no additional resources were allocated
The majority of patients were initiated on ENTRESTO at the nurse-led HF clinics
Prior to initiation, patients underwent a full clinical assessment by the HF nurse specialists, including medication history and review of renal and hepatic function
|
“We hope we have demonstrated that [ENTRESTO] can be appropriately initiated, titrated and managed in this [HF nurse specialist] setting.” |
3. West Midlands – Retrospective multicentre study of 118 symptomatic chronic HFrEF patients in three UK hospital trusts4
This study was published in the Journal of Prescribing Practice, after peer review and technical editing by the publisher. No conflicts of interest were reported.
Objective of the study
A retrospective multicentre study to explore real-life patient data regarding prescribing of ENTRESTO for symptomatic chronic HF patients in three hospitals, in accordance with national guidelines.
Overview
Study | Background | Objective | Findings |
To explore prescribing practices of ENTRESTO at three UK hospitals over a 6-month period in accordance with national NICE guidelines. | Design: A multicentre retrospective study was conducted over a 6-month period of the use on ENTRESTO. The study proforma was adapted from NICE 2016 guidelines. Patient information collected: NYHA functional class, LVEF, and prior HF medication. Data on ENTRESTO treatment were gathered, including side effects, re-admission, discontinuation, and patient demographic data. In addition, blood pressure data, heart rate, eGFR, potassium and sodium levels were collected, as well as patients’ baseline medication (beta-blockers, mineralocorticoid antagonists, diuretics) and devices (implantable cardioverter defibrillator (ICD), cardiac resynchronisation therapy (CRT) and pacemakers (PPM)). |
|
|
Key findings
- Please see the SmPC for further information and initial and following dose adjustments depending on certain conditions
- NICE guidelines recommend ENTRESTO as an option for treating symptomatic chronic HFrEF, only in people: with NYHA class II to IV symptoms and; with a LVEF ≤35%; who are already taking a stable dose of ACEi or ARBs. Treatment with sacubitril/valsartan should be started by a heart failure specialist with access to a multidisciplinary heart failure team. Dose titration and monitoring should be performed by the most appropriate team member as defined in the NICE guideline on chronic heart failure in adults: diagnosis and management.8
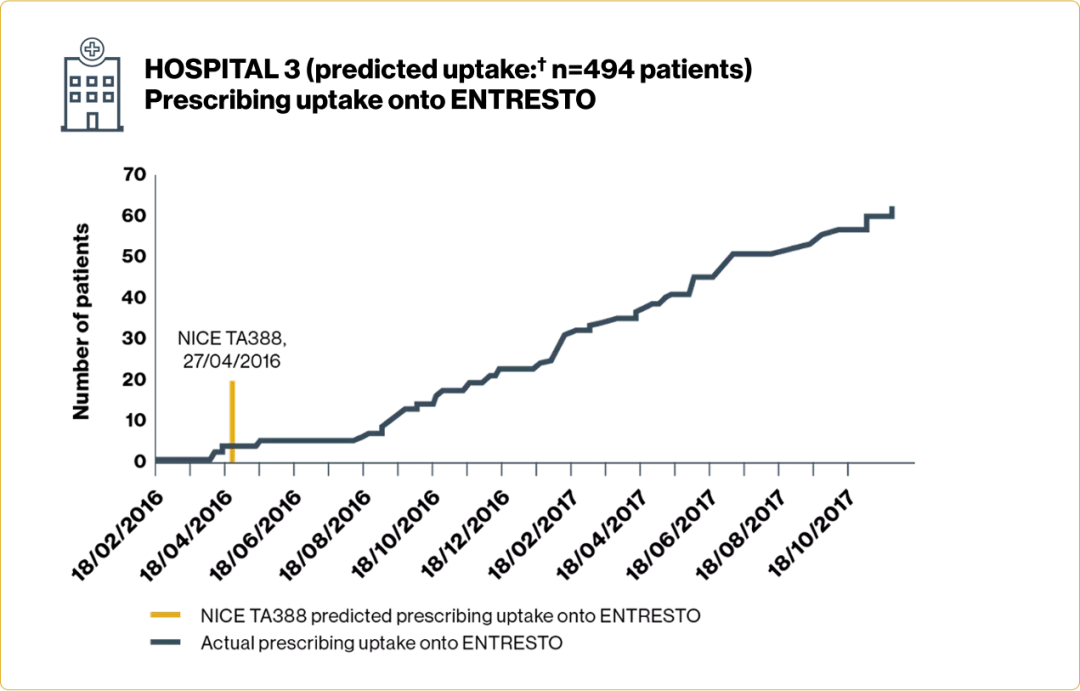
Adherence to NICE guidance* was high across the 3 hospital cohorts studied
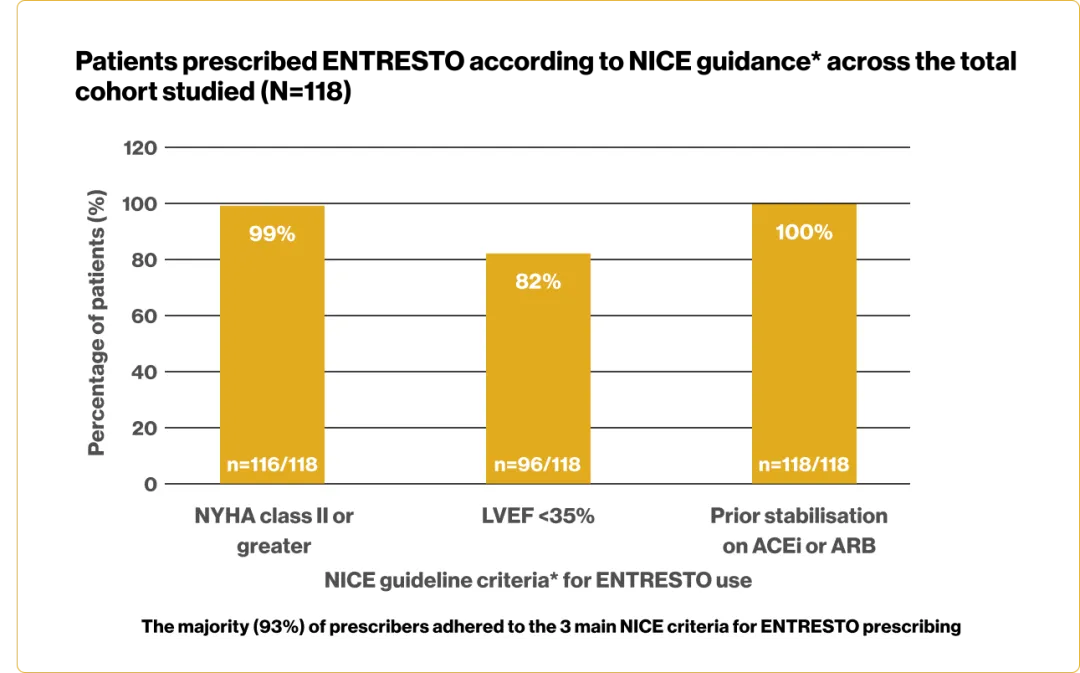
Substantially fewer patients were prescribed ENTRESTO than predicted by the NICE resource tool
Time to initiation and number of patients prescribed ENTRESTO varied across Trusts
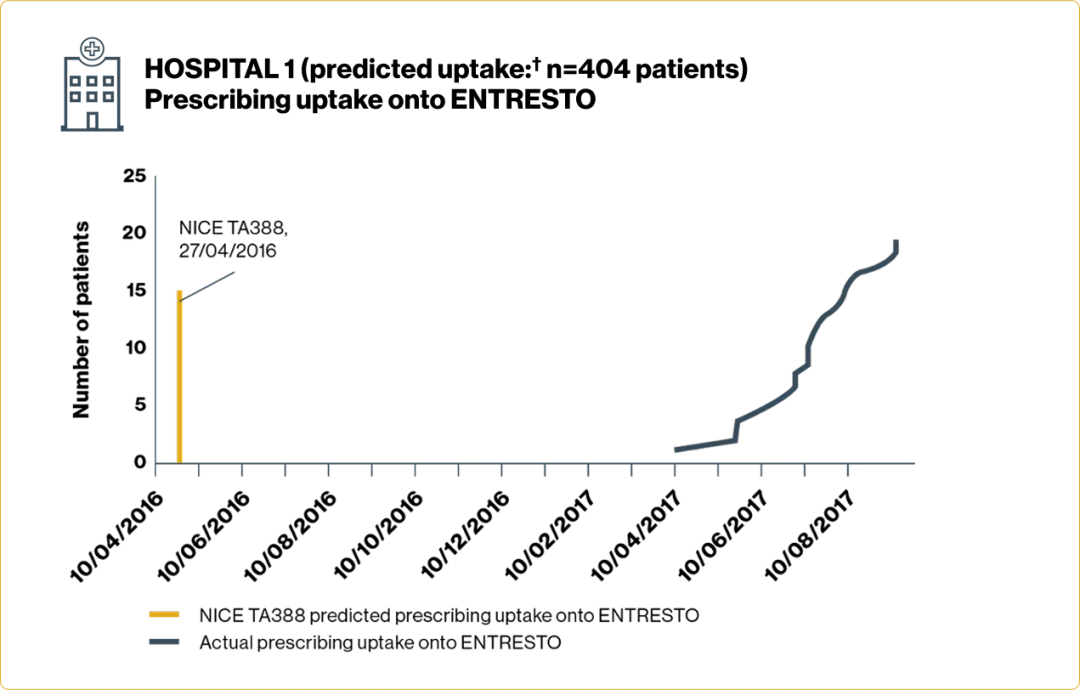
Adapted from Jalal Z, et al. 2019.4
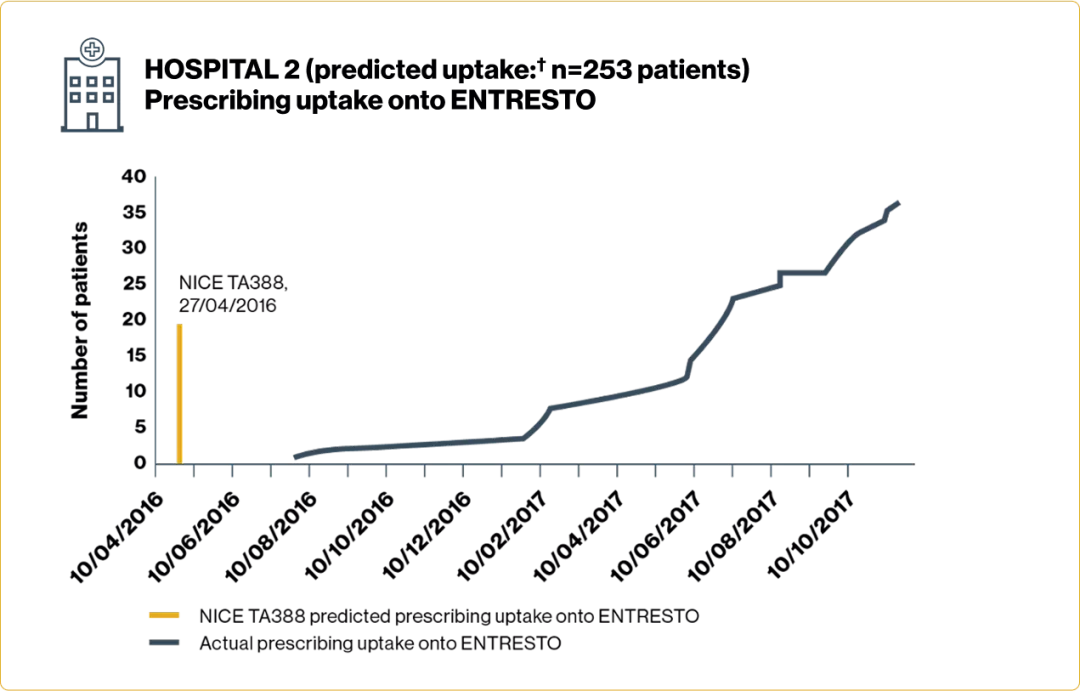
Adapted from Jalal Z, et al. 2019.5
Adapted from Jalal Z, et al. 2019.5
ENTRESTO real-world safety profile‡
The main side effects of ENTRESTO mentioned in the NICE guideline include hypotension, hyperkalaemia and renal impairment.8 Across all Trusts studied, on average 65% (n=76/118) of patients within this study experienced one of the following side effects: dizziness, hypotension, renal impairment and fatigue5
†Based on number of patients anticipated to have met the treatment criteria detailed in NICE TA388 each year.
‡Sample size in this study was small and no clear conclusion could be made due to inconsistency in the documentation of side effects in the 3 hospital databases.
4. University Hospitals Coventry & Warwickshire – Retrospective evaluation of the clinical use of ENTRESTO in a large cardiovascular centre6
Br J Cardiol 2019;26(suppl 1):
Peer-reviewed journal. The sponsored supplement was initiated and funded by Novartis Pharmaceuticals UK Ltd. Editorial control was retained by the authors and editors but Novartis reviewed the supplement for technical accuracy and compliance with relevant regulatory requirements before publication.
Objective of the study
Evaluation of the clinical use of ENTRESTO in a large cardiovascular care centre, in terms of the morbidity and mortality, tolerability and up-titration to its guideline-mandated target dose (97/103 mg BID) in patients switched from ≥4 weeks' ACEi/ARB therapy.
Overview
Study | Background | Objective | Findings |
Early clinical experience with ENTRESTO from a large UK tertiary centre:
| Design: A real-world, retrospective evaluation of the effects of ENTRESTO in 140 patients aged ≥18 years with HFrEF (≤35%), NYHA class II–IV, eGFR >30 mL/min/ 1.73 m2 and ≥4 weeks’ previous treatment with ACEi/ARB. Patients were studied from April 2016 to July 2017 in a nurse-led, MDT-backed HF clinic. | To evaluate the clinical use of ENTRESTO in a large cardiovascular care centre, in terms of morbidity and mortality, tolerability and up-titration to its guideline-mandated target dose (97/103 mg BID) in patients switched from ≥4 weeks ACEi/ARB therapy. |
|
Baseline characteristics
Baseline characteristics | |
Male | 108 (77%) |
Mean age (range), years | 67 (29–89) |
Mean LVEF, % (range) | 23 (8–35) |
NYHA class | |
II | 74 (53%) |
III | 65 (46%) |
IV | 1 (1%) |
Comorbidities | |
Ischaemic heart disease | 46 (33%) |
Atrial fibrillation | 53 (38%) |
Diabetes mellitus | 36 (26%) |
Hypertension | 49 (35%) |
Chronic kidney disease | 39 (28%) |
Medications | |
ACEi | 103 (73%) |
ARB | 38 (27%) |
Beta blocker | 135 (96%) |
MRA | 96 (69%) |
Loop diuretics | 104 (74%) |
Devices | |
Cardiac resynchronisation therapy* | 22 (16%) |
Implantable cardioverter/defibrillator | 8 (6%) |
*Defibrillator/pacemaker.
Data shown are number (%), except where indicated.
Key findings
Up-titration of ENTRESTO in 140 patients initiated on this treatment | |
Event* | N(%) |
Up-titration achieved to 97/103 mg BD | 77 (55%) |
Reduction in loop diuretic dosage | 30 (27%) |
ENTRESTO withdrawn for adverse events | 11 (8%) |
MRA dose reduced due to hyperkalaemia | 2 (2%) |
MRA stopped due to hyperkalaemia | 1 (1%) |
Postural hypotension with drop of SBP >10 mmHg | 44 (31%) |
*Treatment should not be initiated if the serum potassium level is >5.4 mmol/l or with SBP <100 mmHg. If either hypotension or hyperkalaemia occurs during treatment discontinuation should be considered. Please refer to the ENTRESTO SmPC for full guidance.1
If serum potassium level is >5.4 mmol/l discontinuation should be considered.
Up-titration to the target dose was achieved by 55% (n=77/140) patients
ENTRESTO was not tolerated by 11 (8%) patients, mainly due to symptoms of postural hypotension
- Mean SBP fell noticeably between the second and third study visits and remained stable thereafter
- Postural hypotension (SBP reduction >10 mmHg) preventing up-titration to the target dose was observed in 31% (n=44/140) patients at clinic follow-up
Deterioration of renal function in a small proportion of patients did not prevent up-titration to target dose for some, as no progressive impairment of renal function*
eGFR deteriorated by >10 mL/min/1.73 m2 in n=15 (10.7%) patients.
- Five of the fifteen patients achieved up-titration to the target dose
Entresto resulted in low real-world HF mortality
The concomitant use of sacubitril/valsartan with ACE inhibitors is contraindicated. Sacubitril/valsartan must not be started until 36 hours after taking the last dose of ACE inhibitor therapy. ACE inhibitor therapy must not be started until 36 hours after the last dose of sacubitril/valsartan
Event* | N(%) |
All-cause mortality at 6 months (n=140) | 5 (4%)† |
All-cause mortality at 1 year (n=68) | 5 (7%) |
HF admissions at 6 months (n=140) | 8 (6%) |
HF admissions at 1 year (n=68) | 4 (6%) |
*Use of ENTRESTO may be associated with decreased renal function. The risk may be further increased by dehydration or concomitant use of NSAIDs. Down-titration should be considered in patients who develop a clinically significant decrease in renal function. ENTRESTO was not recommended in patients with end-stage renal disease, and concomitant use with aliskiren‐containing medicinal products in patients with diabetes mellitus or in patients with renal impairment (eGFR <60 ml/min/1.73 m2) was contraindicated. Use with caution in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2). Please refer to the ENTRESTO SmPC for full guidance.1
†Two related to HF, three not related to HF.
Patients demonstrated improved LVEF and/or change of at least one NYHA class following ENTRESTO treatment
ENTRESTO treatment resulted in an improvement of at least one NYHA class for 31% (n=43/140) patients
66% of patients (n=23/34 patients who underwent repeat LVEF evaluation post-ENTRESTO treatment) demonstrated improved LVEF with a mean increase from 19% to 27% following ENTRESTO
Of 140 patients treated with ENTRESTO, 7 achieved a normal LVEF of ≥55% from a baseline of severe LV systolic impairment (LVEF ≤35%)7
|
5. Cardiff: Cardiac function, symptoms and QoL with ENTRESTO8
This study was published in the BMJ Open Heart Journal, and was externally peer reviewed. The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. One of the authors, ZY, has received lecture fees and research funding from Novartis.
Objective of the study
Descriptions of the real-world experience of switching stable and optimally medicated patients with HFrEF to ENTRESTO with respect to quality of life and echocardiographic outcomes, and safety.
Overview
Study | Background | Objective | Findings |
Real-world treatment switching to sacubitrll/valsartan in patients with heart failure with reduced ejection fraction:
| Design: Retrospective cohort study of 80 consecutive patients with a diagnosis of stable HFrEF (defined as symptoms and/or signs of HF, NYHA functional classification of ≥II and a LVEF of ≤35% measured by echocardiography) from June 2017 to May 2019. Patients' clinical assessment, biochemistry, echocardiography and QoL were compared pre-treatment switching and post-treatment switching. | Describe the real-world experience of switching stable and optimally medicated patients with HFrEF to ENTRESTO with respect to quality of life and echocardiographic outcomes, and safety. |
|
*ENTRESTO is not recommended in patients with end-stage renal disease and concomitant use with aliskiren‐containing medicinal products in patients with diabetes mellitus or in patients with renal impairment (eGFR <60 ml/min/1.73 m2) is contraindicated. Use with caution in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2).1
Limitations of the study:
Small sample of sequential patients with short follow-up
- Young age group (mean age of 64) may preclude extrapolation to an older cohort, who are more likely to have more advanced disease with a range of comorbidities, such as decline in renal function or autonomic dysfunction
- Lack of comparator group in the study. Patients were already treatment optimised prior to sacubitril/valsartan switch so each effectively acted as his or her own control
Key findings
ENTRESTO resulted in changes in blood pressure, NYHA score and QoL (MLHFQ) in patients with stable HFrEF following a switch from ACEi/ARB to ENTRESTO
n | Pre-switching | Post- switching | Mean difference (SD) | 95% CI | p value | |
NYHA score | 71 | 2.3 | 1.9 | –0.4 (0.63) | –0.6 to –0.2 | <0.001 |
MLHFQ score | 33 | 46 | 38 | –9 (19) | –15 to –2 | 0.016 |
SBP (mmHg) | 68 | 123 | 112 | –10 (14) | –14 to –7 | <0.001 |
DBP (mmHg) | 68 | 72 | 68 | –4 (10) | –6 to –1 | 0.004 |
Changes in cardic structure and function were observed with ENTRESTO in patients with stable HFrEF following a switch from ACEi/ARB to ENTRESTO
n | Pre-switching | Post- switching | Mean difference (SD) | 95% CI | p value | |
LVEF (%) | 49 | 26 | 33 | 7 (10) | 4 to 10 | <0.001 |
LVESD (cm) | 37 | 5.2 | 4.9 | –0.3 (0.8) | –0.6 to –0.08 | 0.013 |
LVEDD (cm) | 48 | 6.0 | 5.7 | –0.3 (0.7) | –0.5 to –0.1 | 0.042 |
ENTRESTO treatment was not associated with a significant change in renal function in patients with stable HFrEF following a switch from ACEi/ARB to ENTRESTO
n | Pre-switching | Post- switching | Mean difference (SD) | 95% CI | p value | |
K+(mmol/L) | 71 | 4.6 | 4.7 | 0.1 (0.40) | –0.01 to 0.20 | 0.054 |
Creatinine (µmol/L) | 71 | 95 | 97 | 2 (14) | –1 to 6 | 0.17 |
eGFR (mL/min/1.73 m2) | 71 | 69 | 67 | –2 (13) | –5 to 1 | 0.23 |
Entresto should not be co-administered with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB). Due to the potential risk of angioedema when used concomitantly with an ACE inhibitor, it must not be started for at least 36 hours after discontinuing ACE inhibitor therapy
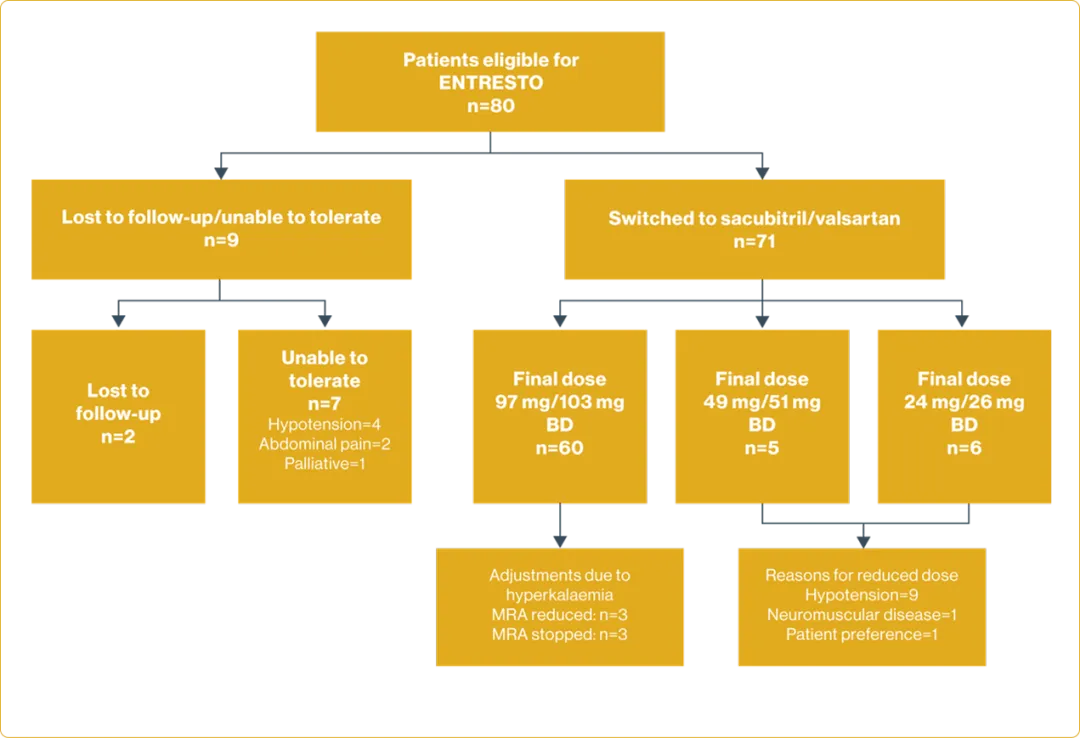
Adapted from Ganesananthan S, et al. 2020.9
ACEi, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BD, twice daily; BP, blood pressure; bpm, beats per minute; CI, confidence interval; CKD, chronic kidney disease; DBP, diastolic blood pressure; DGH, district general hospital; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GP, general practitioner; HCP, healthcare professional; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFrLVEF, heart failure with reduced left ventricular ejection fraction; IV, intravenous; K+, potassium; LV, left ventricular; LVEDD, left ventricular end diastolic diameter; LVEF, left-ventricular ejection fraction; LVESD, left ventricular end systolic diameter; MDT, multidisciplinary team; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MRA, mineralocorticoid receptor antagonist; NICE, National Institute for Health and Care Excellence; NYHA, New York Heart Association; PARADIGM-HF, Prospective Comparison of ARNI with ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure; QoL, quality of life; SBP, systolic blood pressure; SD, standard deviation.
References:
1. ENTRESTO (sacubitril/valsartan) Summary of Product Characteristics.
2. DiMarco A, et al. Heart 2020;106(Suppl. 2):A1–A118.
3. Donnelly E, Patton C. Br J Cardiol 2019;26(Suppl. 1):S22–S24.
4. Jalal Z, et al. J Prescr Pract 2019;1(4):182–192.
5. NICE (2016). Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. TA388. Available at: https://www.nice.org.uk/guidance/ta388/resources/sacubitril-valsartan-for-treating-symptomatic-chronic-heart-failure-with-reduced-ejection-fraction-pdf-82602856425157
6. Ali D, et al. Br J Cardiol 2019;26(Suppl. 1):S9–S14.
7. Pugh J, et al. Audit of de novo initiation of ENTRESTO. Work-based learning 7LMS0157-0206-2020.
8. Ganesananthan S, et al. BMJ 2020;7:e001305.
UK | September 2025 | FA-11286124
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

