
Cosentyx 150 mg provided a favorable and consistent safety profile through 5 years of treatment in patients with AS.2
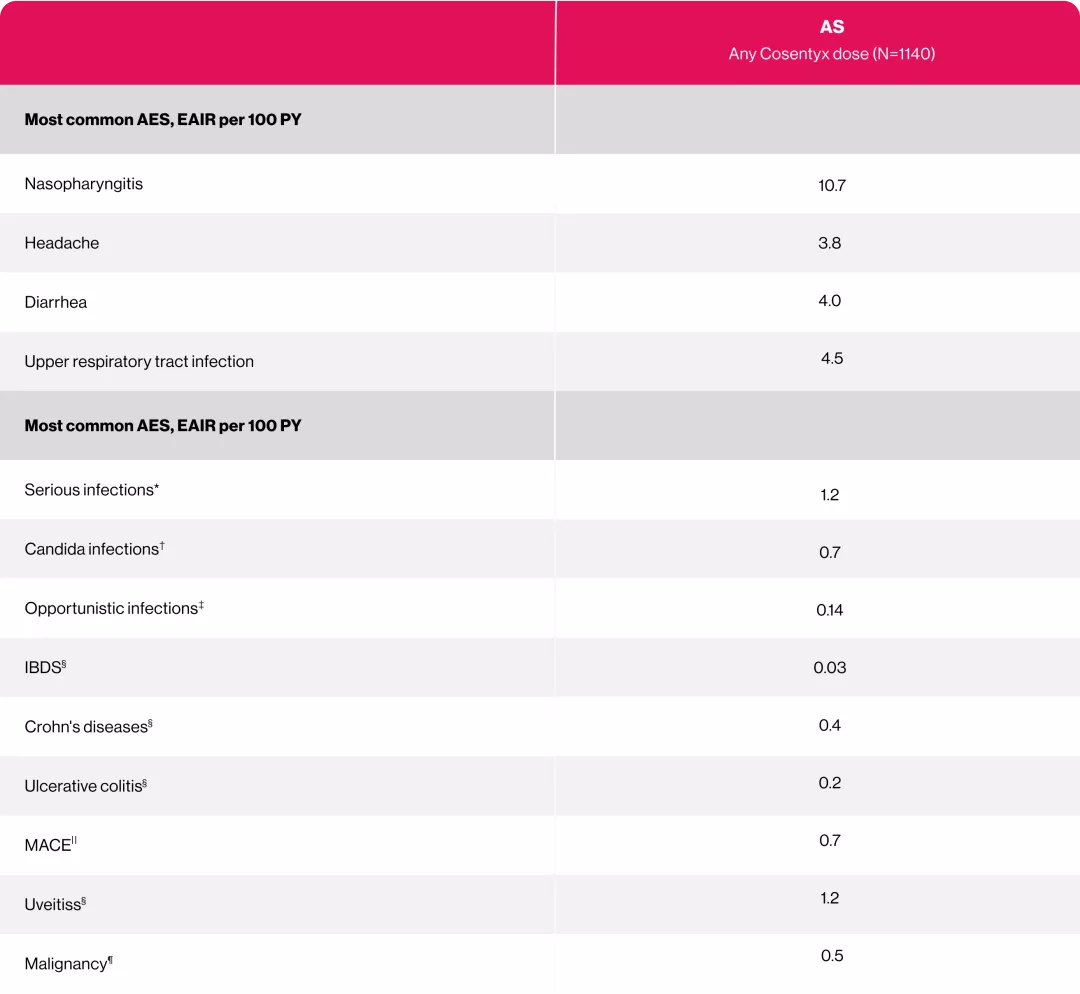
Adapted from reference 3
Safety and experience

Uncommon and easily managed Candida infections3
No increase in incidence of IBD (UC and CD) over time3
No new safety signals, across any indication. 3
Less than 1% immunogenicity4
Cosentyx is not recommended for patients with Inflammatory bowel disease.4
Cosentyx API
Cosentyx API
Footnotes:
*Rates for system organ class.
†Rates for high-level terms.
‡ Opportunistic infections were bronchopulmonary aspergillosis, cytomegalovirus gastroenteritis, gastrointestinal candidiasis, herpes zoster cutaneous disseminated, herpes zoster infection neurological, Mycobacterium avium complex infection, esophageal candidiasis, Pneumocystis jirovecii pneumonia, toxoplasmosis, tuberculosis.
§Rates for preferred terms.
|| Rates for Novartis MedDRA Query terms.
¶Rates for standardized MedDRA Query terms—“malignancies and unspecified tumor.”
AS=ankylosing spondylitis; AEs=adverse events; EAIR=exposure-adjusted incidence rate; IBD=inflammatory bowel disease; MACE=major adverse cardiovascular event; PY=patient-years; MedDRA=Medical Dictionary for Regulatory Activities; CD=Crohn’s disease; IBD=inflammatory bowel disease; UC=ulcerative colitis.
References
Egyptian Drug Authority (EDA) Cosentyx 150,300 mg leaflet approval date: 23/03/2025.
Baraliakos X, Braun J, Deodhar A, Poddubnyy D, Kivitz A, Tahir H, Van den Bosch F, Delicha EM, Talloczy Z, Fierlinger A. Long-term efficacy and safety of secukinumab 150 mg in ankylosing spondylitis: 5-year results from the phase III MEASURE 1 extension study. RMD open. 2019 Sep 1;5(2):e001005.
Gottlieb AB, Deodhar A, Mcinnes IB, Baraliakos X, Reich K, Schreiber S, Bao W, Marfo K, Richards HB, Pricop L, Shete A. Long-term safety of secukinumab over five years in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis and ankylosing spondylitis: update on integrated pooled clinical trial and post-marketing surveillance data. Acta Dermato Venereologica. 2022 Apr 27;102.
Cosentyx Summary of Products characteristics. Available at:
https://www.ema.europa.eu/en/documents/product-information/cosentyx-epar-product-information_en.pdf, Last Accessed: 18/06/2025. Last updated: 10/07/2023.EMA Cosentyx Overview. Available at :https://www.ema.europa.eu/en/documents/overview/cosentyx-epar-medicine-overview_.pdf, Last Accessed: 18/06/2025. Last Updated :03/06/2023.
Approved by Egyptian Drug Authority:BF0424OA4792/092025. Invalidation date: 06/12/2026.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
BF0424OA4792/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
