
Inhibition of IL-17A was shown to be effective in the treatment of ankylosing spondylitis, thus establishing the key role of this cytokine in axial spondyloarthritis.2
IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses.2
Joints | Axial | Skin | Enthesitis | Dactylitis | Nails |
Image

| Image

| Image

| Image

| Image
| Image

|
Cytokines
Chemokines
Mediators of tissue damage
IL-17 contributions to immune and inflammatory disease
Image
| Reduce
|
Image

| Pathogenesis |
| Adapted From reference 2 |
Cosentyx directly inhibits IL-17A in axSpA irrespective of its source3
Joints | Axial | Skin | Enthesitis | Dactylitis | Nails |
Image

| Image

| Image

| Image

| Image

| Image

|
Adapted From reference 3
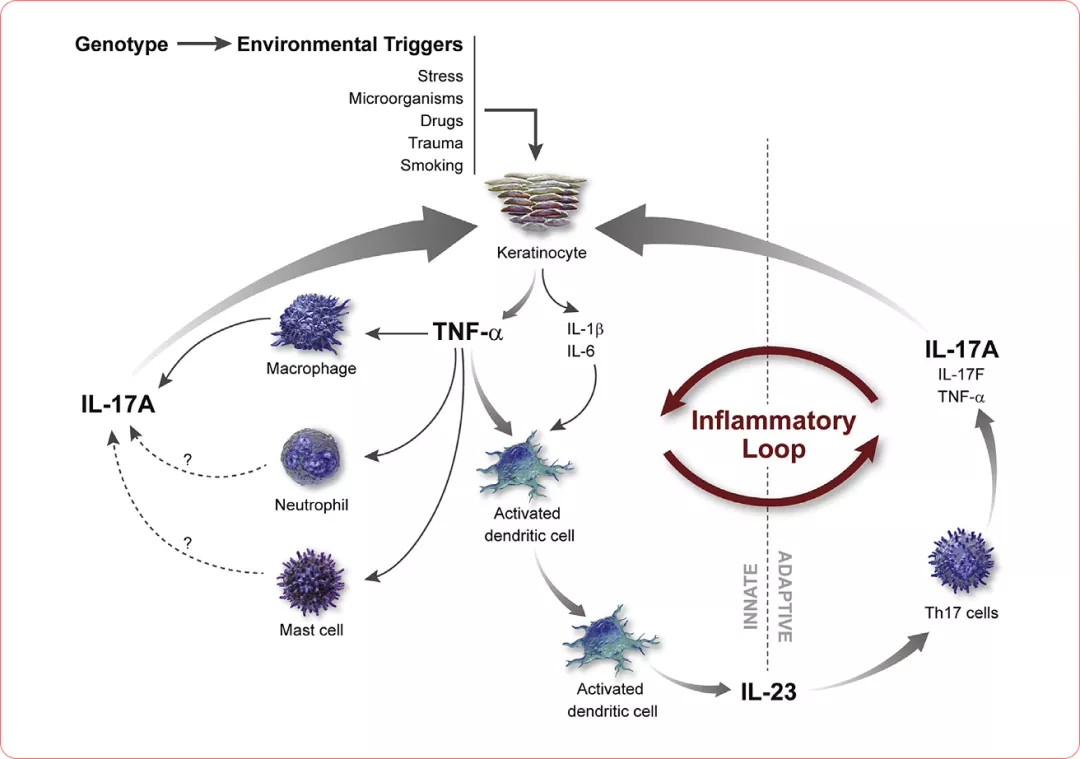
Current model of psoriasis pathogenesis. The T-helper (Th)1 arm shown in Fig 2 may still play a role, but available evidence suggests that the Th17 arm is more important.3
Adapted From reference 4
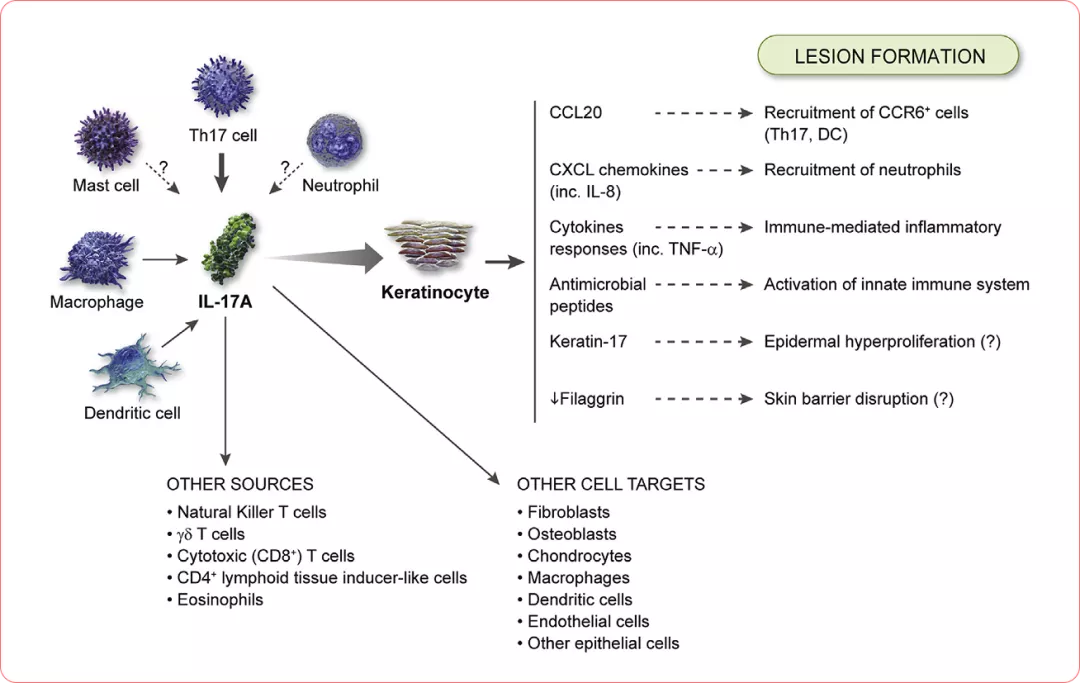
Cellular sources and targets in psoriasis. T-helper (Th)17 cells are the key cellular sources, and keratinocytes are the key cellular target of interleukin (IL)-17A. Neutrophils stain positive for IL-17, but production of the protein has yet to be confirmed. Similarly, some evidence suggests that mast cells produce IL-17A, but further investigation is required.4
Increased levels of IL-17A are found in the affected tissues of patients with axSpA, PsA, PsO, HS1
Image

| Across 8 indications1 |








Cosentyx API
Cosentyx API
Footnotes:
axSpA=axial spondyloarthritis and includes AS (ankylosing spondylitis) and nr-axSpA (non-radiographic axial spondyloarthritis);
IL=interleukin; MOA=mechanism of action; MOD=mechanism of disease; PsA=psoriatic arthritis; PsO=plaque psoriasis; Th17=T helper 17; TNF=tumor necrosis factor; CCL=Chemokine (C-C motif) ligand; CXCL, chemokine (C-X-C motif) ligand; DC= dendritic cell; HS=hidradenitis suppurativa.
References
Egyptian Drug Authority (EDA) Cosentyx 150,300 mg leaflet approval date: 23/03/2025.
Cosentyx Summary of Products characteristics. Available at:
https://www.ema.europa.eu/en/documents/product-information/cosentyx-epar-product-information_en.pdf, Last Accessed: 18/06/2025. Last updated: 10/07/2023.Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. Journal of the American Academy of Dermatology.2014 Jul 1;71(1):141-50.
Kehl AS, Corr M, Weisman MH. Enthesitis: new insights into pathogenesis, diagnostic modalities, and treatment.Arthritis & rheumatology (Hoboken, NJ). 2016 Feb;68(2):312.
Approved by Egyptian Drug Authority:BF0424OA4792/092025. Invalidation date: 06/12/2026.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
BF0424OA4792/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |



