
The simplicity of ONE monthly maintenance injection, regardless of dose1
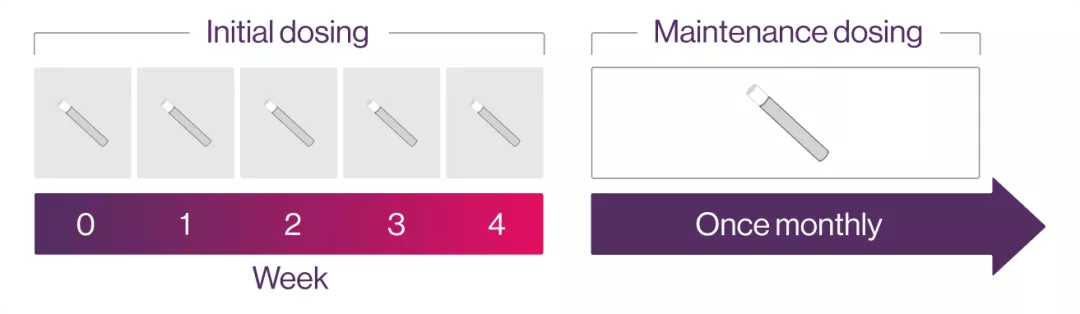


axSpA: The recommended initial dose is 150 mg for both nr-axSpA and AS. followed by monthly maintenance dose.1
Each 300 mg dose is given as one subcutaneous injection of 300 mg or two injections of 150 mg.1
Cosentyx API
Cosentyx API
Footnotes:
AS=ankylosing spondylitis; axSpA=axial spondyloarthritis; nr-axSpA=non-radiographic axial spondyloarthritis; s.c.=subcutaneous.
References
Egyptian Drug Authority (EDA) Cosentyx 150,300 mg leaflet approval date: 23/03/2025.
Approved by Egyptian Drug Authority:BF0424OA4792/092025. Invalidation date: 06/12/2026.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
BF0424OA4792/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
