
Cataflam® 75mg/3mL solution for injection
Indication
Cataflam® 75 mg/3 mL pronounced analgesic effect in moderate and severe pain.1
Cataflam® 75 mg/3 mL (diclofenac potassium) ampoule is indicated in short-term treatment in the following acute conditions:1
Image

| Post-traumatic pain, inflammation, and swelling e.g., due to sprains |
Image

| Postoperative pain, inflammation, and swelling e.g., following dental pain or orthopedic surgery. |
Dosage
Image

| The dose is generally one 75 mg ampoule daily.1 |
Image

| In severe cases (e.g., colic), the daily dose can be increased to two injections of 75 mg.1 |
Pharmacokinetics
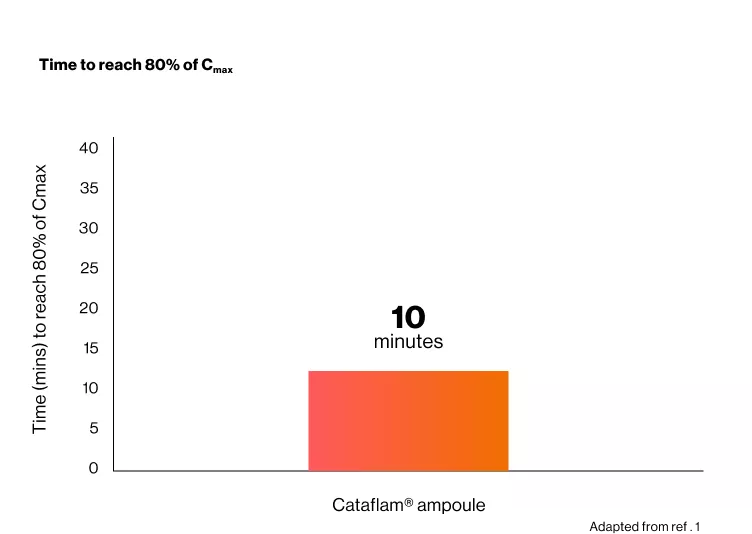
- Cataflam® 75 mg/3 mL takes only 10 minutes to reach 80% of the mean maximum plasma concentration.1
- In the presence of inflammation, it relieves both spontaneous pain and pain on movement.1
For Cataflam® 75 mg/3 mL Abbreviated prescribing information
For Cataflam® 75 mg/3 mL Abbreviated prescribing information
References
Cataflam® 75mg/3ml solution for injection Egyptian Drug Authority approved insert leaflet. Approval Date: 23/9/2024
Approved by Egyptian Drug Authority: HF0082OA4734/092025. Invalidation date: 01/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0082OA4734/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
