
Catafast 50mg granules
Indication
Catafast® 50mg (diclofenac potassium) is indicated for post-operative inflammation and pain following orthopedic surgery.1
Dosage
The recommended dose is 50 mg 2-3 times daily in divided doses.1
Efficacy
In a double-blind study, the investigator rated diclofenac dispersible as excellent for 55% of the patients, good for 35%, and fair for only 10%. In comparison, ketorolac was rated as excellent for 16% of patients, good for 72%, and fair for 12%.2
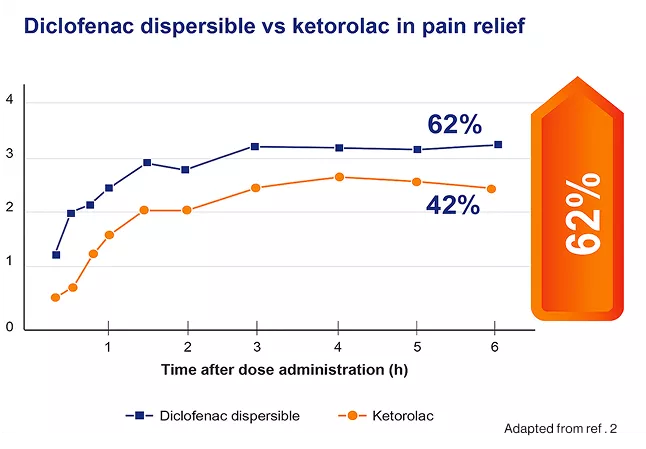
Diclofenac dispersible demonstrated significantly superior efficacy over ketorolac in reducing postoperative pain starting from the 15-minute time point post-dose, with an estimated treatment difference of 9.0 mm (p = 0.01) in the intent-to-treat dataset and 8.13 mm (p = 0.005) in the acceptable patient dataset. After six hours, 62% of patients treated with diclofenac (n = 29) experienced complete pain relief compared to 42% of those treated with ketorolac (n = 26).2
Pharmacokinetics
- Catafast® is quickly absorbed due to its buffering formulation.1*
- Mean peak plasma concentrations of 1.6 micrograms attained after 5 to 20 minutes after ingestion of one sachet of 50 mg.2
* Combined with potassium salt of diclofenac.1
For Catafast® Abbreviated prescribing information
For Catafast® Abbreviated prescribing information
References
Catafast® 50 mg Egyptian Drug Authority approved insert leaflet, Approval date:7/11/2023.
Fineschi G, Tamburrelli FC, Francucci BM, Pisati R. Oral Diclofenac Dispersible Provides a Faster Onset of Analgesia than Intramuscular Ketorolac in the Treatment of Postoperative Pain. Clinical Drug Investigation. 1997;13:1–7
Approved by Egyptian Drug Authority: HF0082OA4734/092025. Invalidation date: 01/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0082OA4734/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
