
Arthrofast 150mg modified release tablets
Indication
Arthrofast® 150mg – Symptomatic treatment of pain and inflammation in case of:1
- Chronic joint inflammation, especially rheumatoid arthritis (chronic polyarthritis)
Dosage
|
| Image

|
Efficacy
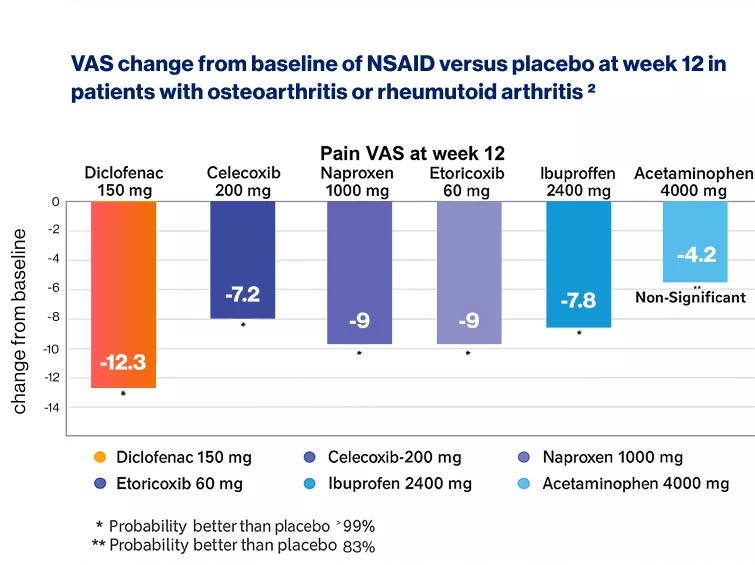
VAS: Visual analogue scale, NSAID: Non-steroidal anti-inflammatory drugs
Diclofenac (150 mg/day) is likely to be more efficacious in alleviating osteoarthritis (OA) or rheumatoid arthritis (RA) pain compared to other treatments at week 12.2
A network meta-analysis involving 146,524 patients across 176 studies evaluated the efficacy, safety, and tolerability of various treatments and found that diclofenac was more likely to be effective in relieving OA or RA pain than celecoxib (200 mg/day), naproxen (1000 mg/day), and ibuprofen (2400 mg/day), while demonstrating similar efficacy to etoricoxib (60 mg/day)2
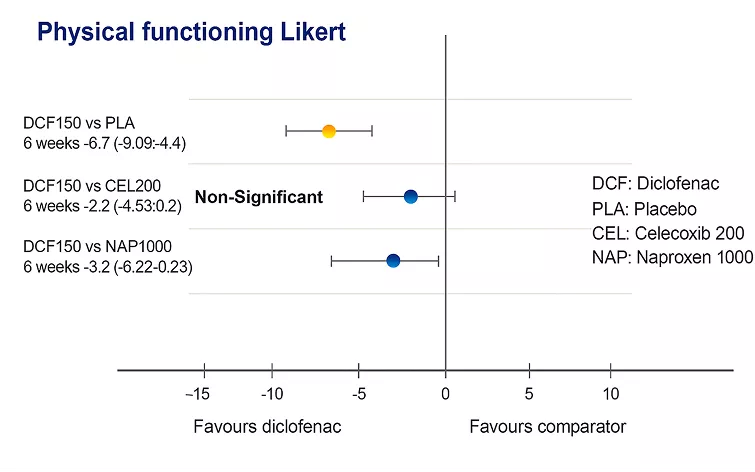
Arthrofast® showed an improvement in physical functioning in 6 weeks in patients with osteoarthritis or rheumatoid arthritis.2
Arthrofast® (diclofenac 150 mg/day) has been shown to be more effective in improving physical functioning compared to both naproxen and placebo, based on Likert assessment, over a treatment period of six weeks.2
OA: Osteoarthritis, RA: Rheumatoid arthritis.
For Arthrofast® Abbreviated prescribing information
For Arthrofast® Abbreviated prescribing information
References
Arthrofast® 150mg. Egyptian Drug Authority approved insert leaflet, Approval date:22/01/2024
Van Walsem A, Pandhi S, Nixon RM, Guyot P, Karabis A, Moore RA. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta-analysis. Arthritis research & therapy. 2015;17(1):1-8.
Approved by Egyptian Drug Authority: HF0082OA4734/092025. Invalidation date: 01/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0082OA4734/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
