
Cataflam 50mg sugar-coated tablets
Indication
Cataflam® 50 mg sugar- coated tablets can relieve symptoms of inflammation such as pain and swelling by blocking the synthesis of the molecules (prostaglandins) responsible for inflammation, pain and fever.1
Dosage
Image

| Adults: 100-150 mg per day.1 |
Image

| For milder cases and children over 14 years of age 50-100 mg per day is usually sufficient.1 |
Efficacy
Cataflam® 50mg was found to be more effective than aspirin 650 mg and had a longer duration of effect (at 30 minutes, 4 and 8 hours) in severe pain.2
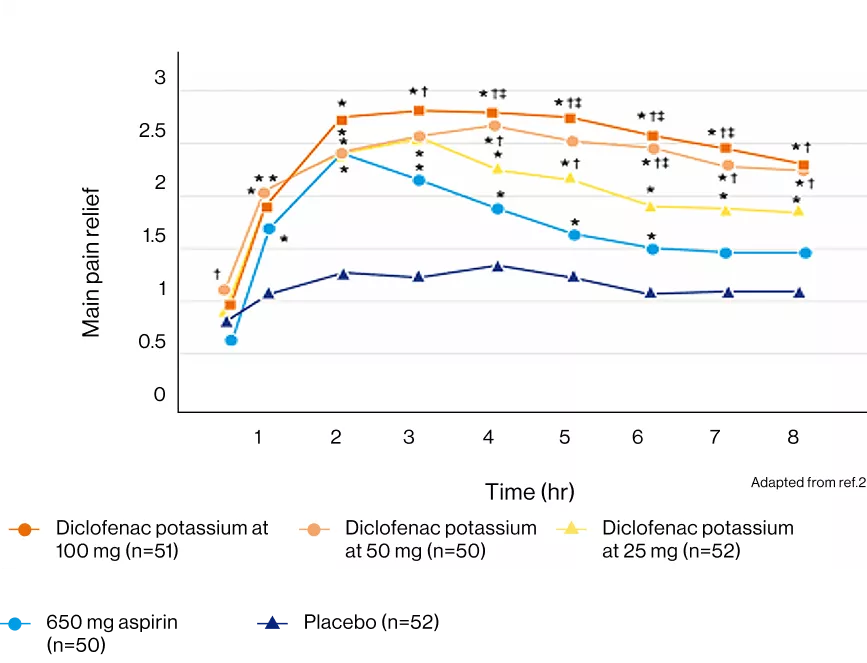
In a systematic review to evaluate the analgesic efficacy and safety of oral diclofenac in the treatment of acute postoperative pain, the proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with diclofenac 100 mg was 56%.3
Fisher's protected least significant difference (LSD) test:
*significantly superior to placebo, LSD p < 0.05
†significantly superior to 650 mg of aspirin, LSD p < 0.05
‡significantly superior to 25 mg of diclofenac LSD p <= 0.05

NNT= number needed to treat to benefit
For Cataflam® 50 mg Abbreviated prescribing information
For Cataflam® 50 mg Abbreviated prescribing information
References
Cataflam® 50 mg Egyptian Drug Authority approved insert leaflet, Approval date: 26/12/2024.
Olson NZ, Sunshine A, Zighelboim I, et al. Onset and duration of analgesia of diclofenac potassium in the treatment of postepisiotomy pain. Am J Ther. 1997;4(7- 8):239-246.
Derry P, Derry S, Moore RA, McQuay HJ. SIngle dose oral diclofenac for acute postoperative pain in adults. Crochane Database of Systemic Reviews. 2009)
Approved by Egyptian Drug Authority: HF0082OA4734/092025. Invalidation date: 01/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0082OA4734/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
