
Voltaren 75mg/3ml ampoules
Voltaren® 75mg/3mL ampoule is indicated for
Post-traumatic and post-operative pain, inflammation and swelling.1
Dosage

Following i.m. injection of 75 mg diclofenac, mean peak plasma concentrations of 2.5 μg/ml are attained after approximately 20 minutes1


Efficacy
Postoperative pain was significantly (P < 0.05) milder in patients receiving Voltaren® IV vs placebo.2
The incidence of postoperative pain in the oral region in patients undergoing oral surgery or restorative dentistry under isoflurane anaesthesia preceded by diclofenac sodium or saline
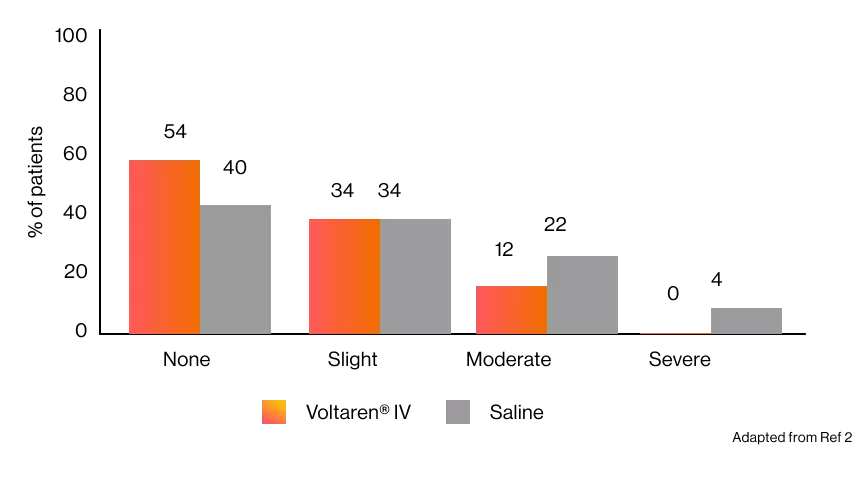
For Voltaren® Amp. Abbreviated prescribing information
For Voltaren® Amp. Abbreviated prescribing information
References
Voltaren 75mg/3ml solution for Injection Egyptian Drug Authority (EDA) approved insert leaflet. Date of approval: 10/02/2025.
Valanne J, Korttila K, Ylikorkala O. Intravenous diclofenac sodium decreases prostaglandin synthesis and postoperative symptoms after general anaesthesia in outpatients undergoing dental surgery. Acta anaesthesiologica scandinavica. 1987 Nov;31(8):722-7.
Approved by Egyptian Drug Authority: HF0082OA4733/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0082OA4733/082025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
