
Cataflam 75mg/3ml solution for injection
Cataflam®75mg/3mL ampoule has been found to exert a pronounced analgesic effect in moderate and severe pain. It is indicated in the short-term treatment of Post-operative pain, inflammation, and swelling e.g., following dental pain or surgery1
Cataflam®75mg/3mL ampoule has a “rapid pain relief.“1
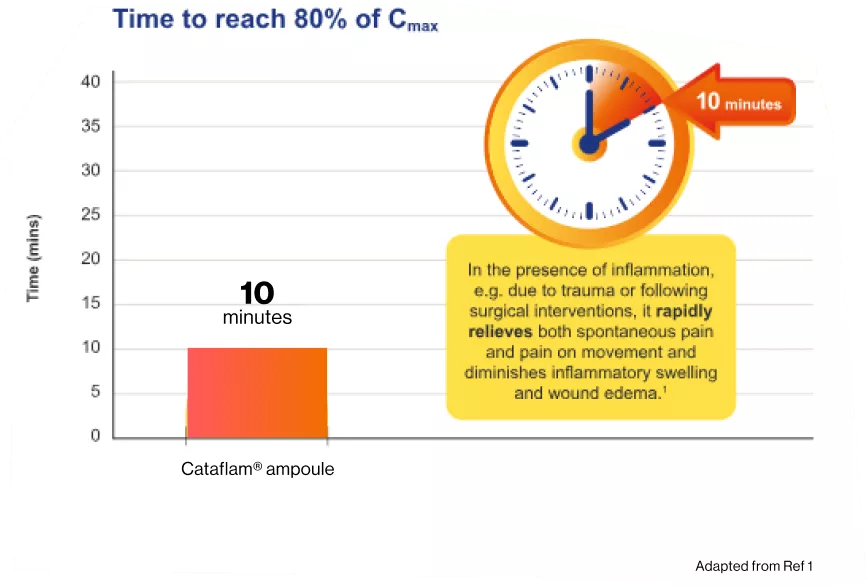
In the presence of inflammation, e.g., due to trauma or following surgical interventions, it
rapidly relieves both spontaneous pain and pain on movement and diminishes inflammatory
swelling and wound edema.
Dosage
Image

| The dose is generally one 75 mg ampoule daily, given by deep intragluteal injection into the upper outer quadrant using aseptic technique 1 |
Efficacy
In a double-blind study comparing the efficacy and safety of ketorolac with that of Diclofenac 75 mg and placebo for patients undergoing removal of impacted third molar teeth, diclofenac 75 mg provided Significant pain relief and no adverse events were recorded when administered following oral surgery.2
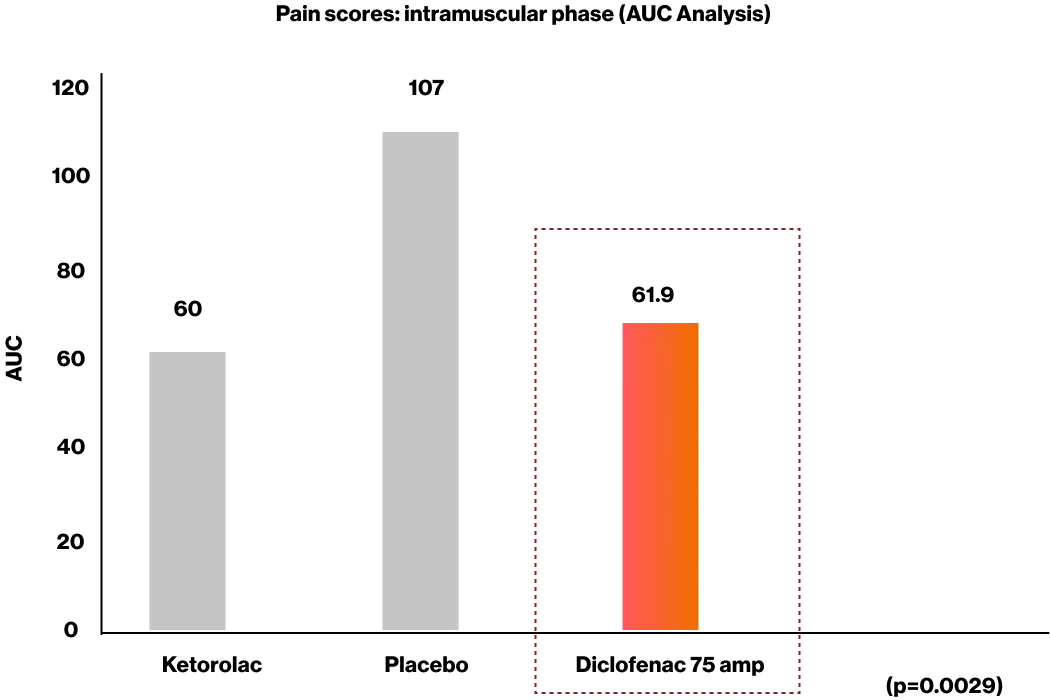
Results showed that both analgesic preparations were more effective than placebo (p=0.0029) in pain relief.2
Cataflam®75mg/3mL ampoule take Only 10 minutes to reach 80% of the mean maximum plasma concentration.¹
For Cataflam® 75 mg/3 mL Abbreviated prescribing information
For Cataflam® 75 mg/3 mL Abbreviated prescribing information
References
Cataflam® 75mg/3ml solution for injection Egyptian Drug Authority approved insert leaflet.Date of approval: 23/9/2024.
Walton GM, Rood JP, Snowdon AT, et al. Ketorolac and diclofenac for postoperative pain relief following oral surgery. Br J Oral Maxillofac Surg. 1993;31(3):158-160.
Approved by Egyptian Drug Authority: HF0082OA4733/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0082OA4733/082025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
