
Catafast® Sachets
Catafast® is indicated for post operative pain, inflammation and swelling following dental surgery1
Dosage
Image

| 2-3 sachets of Catafast® (100-150 mg Per day).1 |
Image

| Mean peak plasma concentrations of 1.6 micrograms (ug) are attained within 5 to 20 minutes after ingestion of one sachet of 50 mg1 |
Image
| Catafast® (combined with potassium salt of diclofenac) has a quick absorption rate due to its dynamic buffering formulation 2 |
Image

| More than 7 out of 10 patients treated with Catafast® after dental surgery had a global evaluation of ‘very good’ or ‘good’ versus 3 out of 10 with placebo3 |
Catafast® shows an 80% reduction in postoperative dental patients after 2 hours post dose3
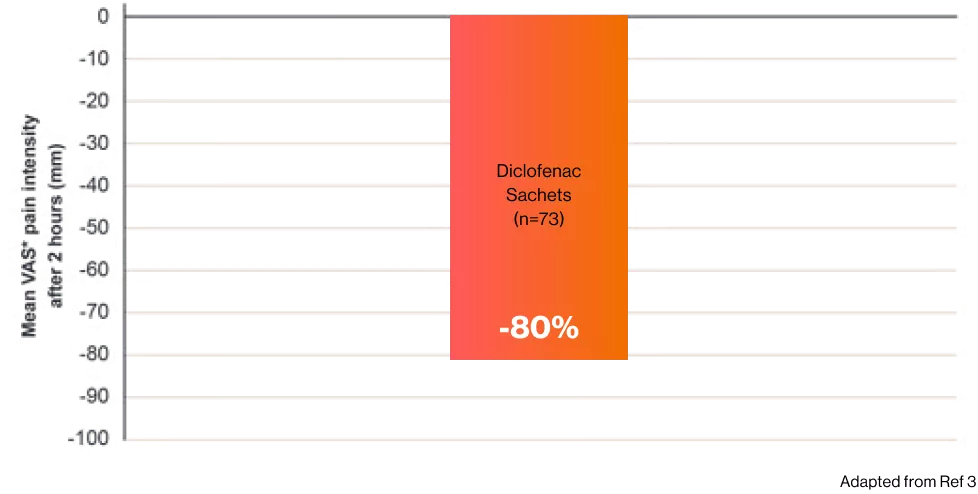
Catafast® sachets was significantly superior to placebo, (p < 0.0001)3
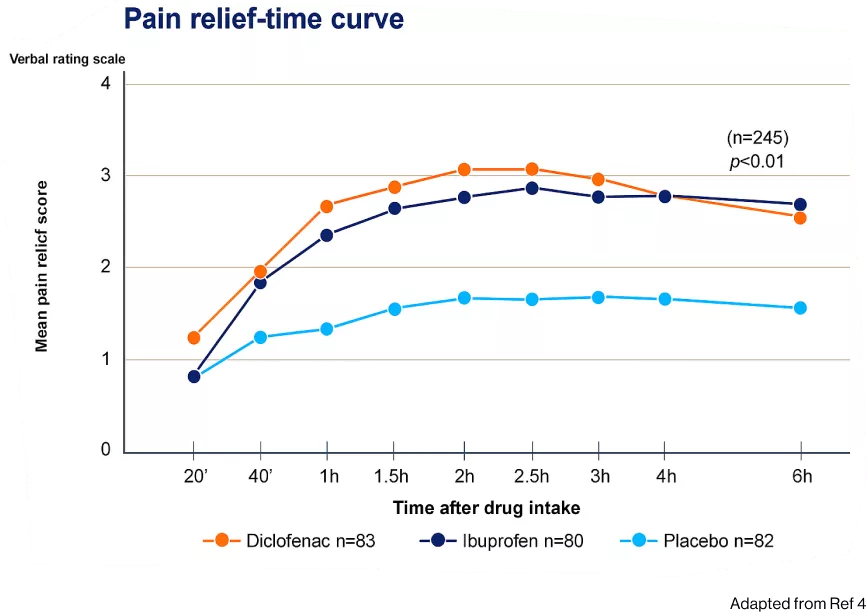
Mean pain intensity scores showed that Catafast® was significantly superior to ibuprofen at 20 minutes, but not thereafter (p<0.05), and both NSAIDs led to a sustained decrease in mean scores over the 6-hour observation period4
For Catafast® Abbreviated prescribing information
For Catafast® Abbreviated prescribing information
References
Catafast ® 50 mg Egyptian Drug Authority approved insert leaflet 7/11/2023.
Chen C, Bujanover S, Kareht S, et al. Differential pharmacokinetics of diclofenac potassium for oral solution vs immediate-release tablets from a randomized trial: effect of fed and fasting conditions. Headache. 2015;55(2):265-275.
Hofele CM, Gyenes V, Daems LN, et al. Efficacy and tolerability of diclofenac potassium sachets in acute postoperative dental pain: a placebo-controlled, randomised, comparative study vs. diclofenac potassium tablets. Int J Clin Pract. 2006;60(3):300-307.
Bakshi R, Frenkel G, Dietlein G, et al. A placebo-controlled comparative evaluation of diclofenac dispersible versus ibuprofen in postoperative pain after third molar surgery. J Clin Pharmacol. 1994;34(3):225-230.
Approved by Egyptian Drug Authority: HF0082OA4733/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0082OA4733/082025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
