
Cataflam 50mg sugar-coated tablets
Rapid pain relief is possible for your patients1
- Indicated for inflammation and pain, e.g. following dental surgery.1
- Have pronounced analgesic, anti-inflammatory, and antipyretic properties.1
Dosage
Image

| Adults: |
Image

| For milder cases and children over 14 years of age: 50-100 mg per day is usually sufficient.1 |
- Have a rapid onset of action that starts 20 to 60 minutes after ingestion of one 50mg tablet1
Efficacy
- Post endodontic pain prevention is important for both patients and clinicians.2
- Postoperative pain incidence is reported to be 40% within 24–48 hours with a range up to 58%.2
- Cataflam®50 mg Tablets showed significantly less pain intensity than the placebo 48 hours postoperatively (P=0.028)2
- Another significant decrease in pain level occurred with Cataflam® from 24 to 48 hours (P=0.002), which did not occur with the placebo (P=1.000)2
- Less patients had moderate to severe pain with Cataflam® (2.9%) than the placebo (20.6%) at 48 hours (P=0.024)2

86% Of Cataflam® 50 mg patients reported no pain perception 36 hours after extraction3
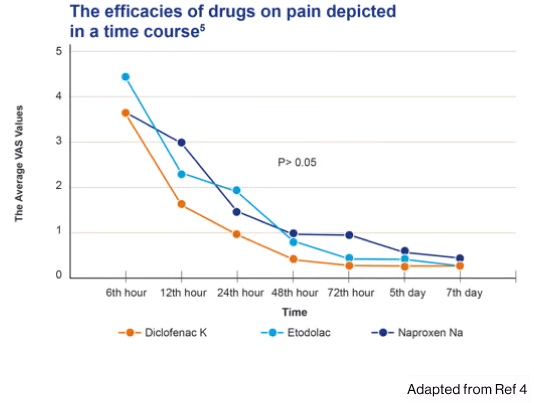
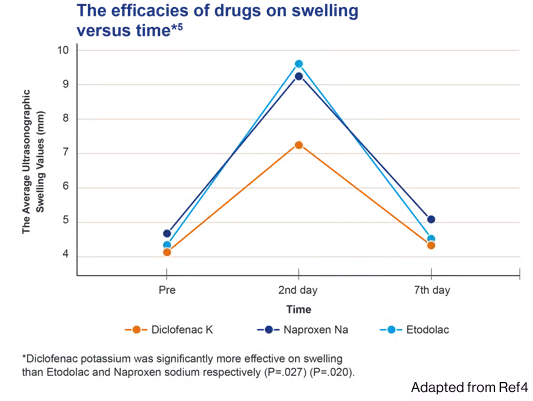
- In a study comparing the effect of 3 NSAIDs on pain, swelling, and trismus following third molar surgery, Cataflam® was significantly more effective on swelling than etodolac and naproxen sodium respectively (P=.027) (P=.020)4
- The present study aligned the drugs as diclofanac potassium> naproxen sodium = etodolac in terms of reducing the postoperative third molar surgery sequelae4
For Cataflam® 50 mg Abbreviated prescribing information
For Cataflam® 50 mg Abbreviated prescribing information
References
Cataflam® 50mg Egyptian Drug Authority approved insert leaflet. Date of approval: 26/12/2024.
Al-Rawhani AH, Gawdat SI, Amin SA. Effect of diclofenac potassium premedication on postendodontic pain in mandibular molars with symptomatic irreversible pulpitis: a randomized placebo-controlled double-blind trial. Journal of Endodontics. 2020 Aug 1;46(8):1023-31.
Nourwali I, Namnakani A, Almutairi M, Alaufi A, Aljohani Y, Kassim S. Loxoprofen sodium versus diclofenac potassium for post-dental extraction pain relief: a randomized, triple-blind, clinical trial. Dentistry Journal. 2019 Dec 25;8(1):2.
Akbulut N, Üstüner E, Atakan C, et al. Comparison of the effect of naproxen, etodolac and diclofenac on postoperative sequels following third molar surgery: a randomised, double-blind, crossover study. Med Oral Patol Oral Cir Bucal. 2014;19(2):e149-e156.
Approved by Egyptian Drug Authority: HF0082OA4733/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0082OA4733/082025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
