
Symptoms Control
MPN’s symptomatic burden affects patient’s health-related quality of life and daily routines1
Some of Prevalent symptoms in MF2
Fatigue
98.9%
Inactivity

76.5%
Early satiety
75.5%
Insomnia
73.9%
Concentration difficulties

72.5%
Abdominal discomfort
71.7%
Abdominal pain
63%
Weight loss
(Constitutional symptom)

48.9%
Adapted from ref2
Symptom burden impairs activity and productivity1
Patients experiencing effect of symptoms upon daily activities
Cancelling planned
activitiesa

41%
n=207
Staying all/most
of day in beda

32%
n=207
Requiring reduced
working hoursb

59%
n=119
Taking days off work
due to sicknessa
29 %
n= 52
a In the preceding 30 days
b Respondents employed full- or part-time only
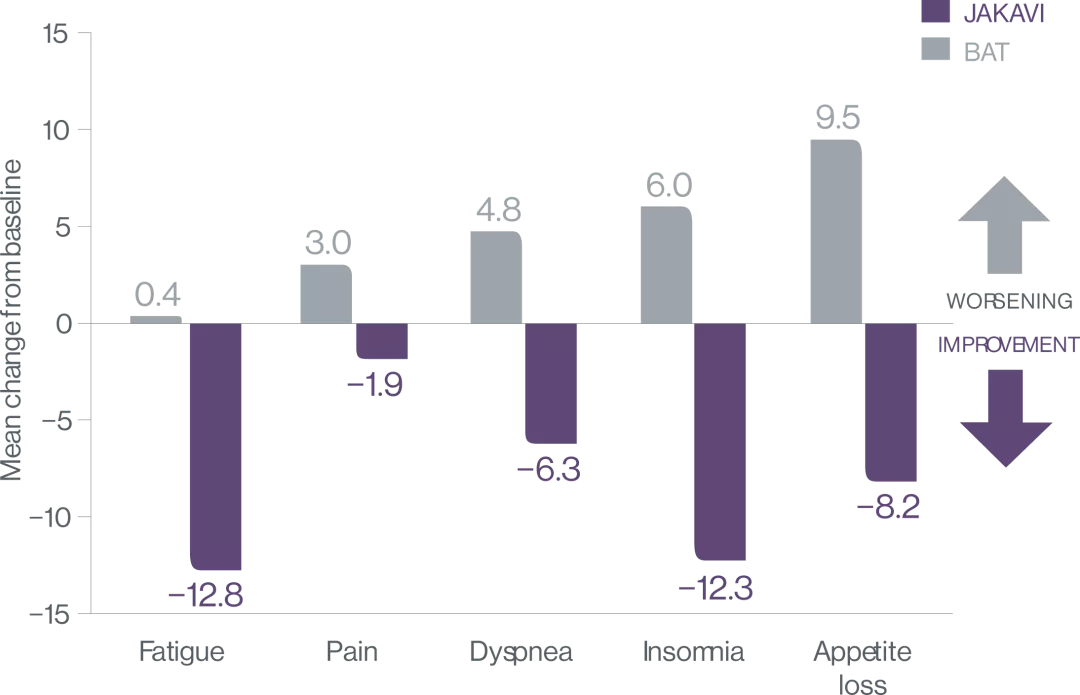
Patients receiving ruxolitinib show improvements in myelofibrosis-associated symptoms3
COMFORT-II: Mean change in EORTC QLQ-C30 symptom scores3
MPNs: Philadelphia-chromosome negative myeloproliferative neoplasms
For JAKAVI-MF Abbreviated prescribing information
For JAKAVI-MF Abbreviated prescribing information
References
Mesa R, Miller CB, Thyne M,et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN Landmark survey. BMC cancer. 2016;16(1):1-0.
Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF):
International Prospective Validation and Reliability Trial in 402 patients. Blood. 2011; 01-328955Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. New England Journal of Medicine. 2012 Mar 1;366(9):787-98.
Approved by Egyptian Drug Authority: HF0424OA4703/102025. Invalidation date: 17/12/2025.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0424OA4703/102025 17/12/2025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
