
Dosing
Deliver maximum benefits by treating at the right dose1
The dose starts from 10 mg linked with platelet count1
Platelet count | 50,000 to <100,000/mm3 | 100,000 to <200,000/mm3 | > 200,000/mm3 |
|---|---|---|---|
Dose | SVG
10 mg orally | SVG
15 mg orally | SVG
20 mg orally |
Tailor their treatment for optimal outcomes1
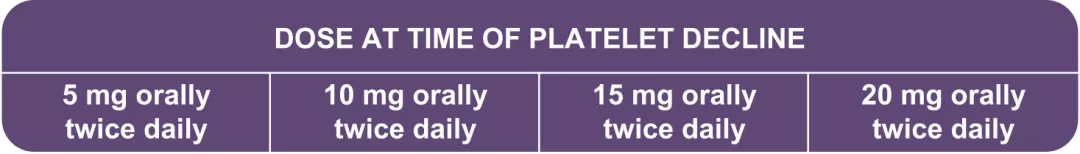
50,000 to <75,000/mm3 | No change | 5 mg orally | 5 mg orally | 5 mg orally |
75,000 to <100,000/mm3 | No change | No change | 10 mg orally | 10 mg orally |
100,000 to <125,000/mm3 | No change | No change | No change | 15 mg orally |
Treat at diagnosis with JAKAVI1
JAKAVI is a potent JAK1 and JAK2 inhibitor1
JAKAVI acts on both JAK1 and JAK2 to inhibit overactive JAK/STAT signalling in MF1.
JAKAVI binds to the kinase domain of JAK1 and JAK2 and inhibits JAK1 and JAK2 signalling regardless of the JAK2V617F mutation status1.
JAKAVI inhibits JAK stimulation of STAT and downstream effects on cellular proliferation1.
For JAKAVI-MF Abbreviated prescribing information
For JAKAVI-MF Abbreviated prescribing information
References
Egyptian drug authority(EDA). Jakavi Approved Leaflet approval date 18-03-2024
Approved by Egyptian Drug Authority: HF0424OA4703/102025. Invalidation date: 17/12/2025.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0424OA4703/102025 17/12/2025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
