
Spleen Reduction
Each 10% reduction in spleen length was associated with 9% reduction in risk of death (HR=0.91; 95%CI: 0.84-0.99; P=0.02)1
Reduction in spleen size was predictive for improved overall survival2

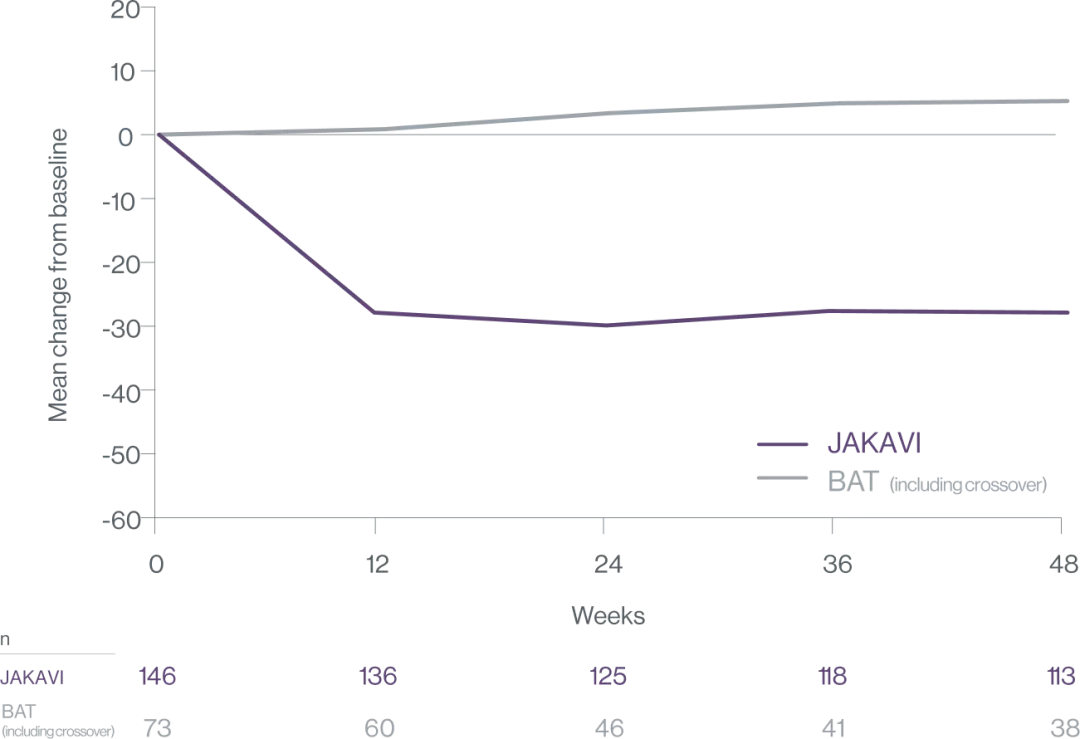
Mean percentage change in spleen volume3
|
Early intervention with JAKAVI may result in higher rates of spleen response3
Predictors of spleen response with JAKAVI1
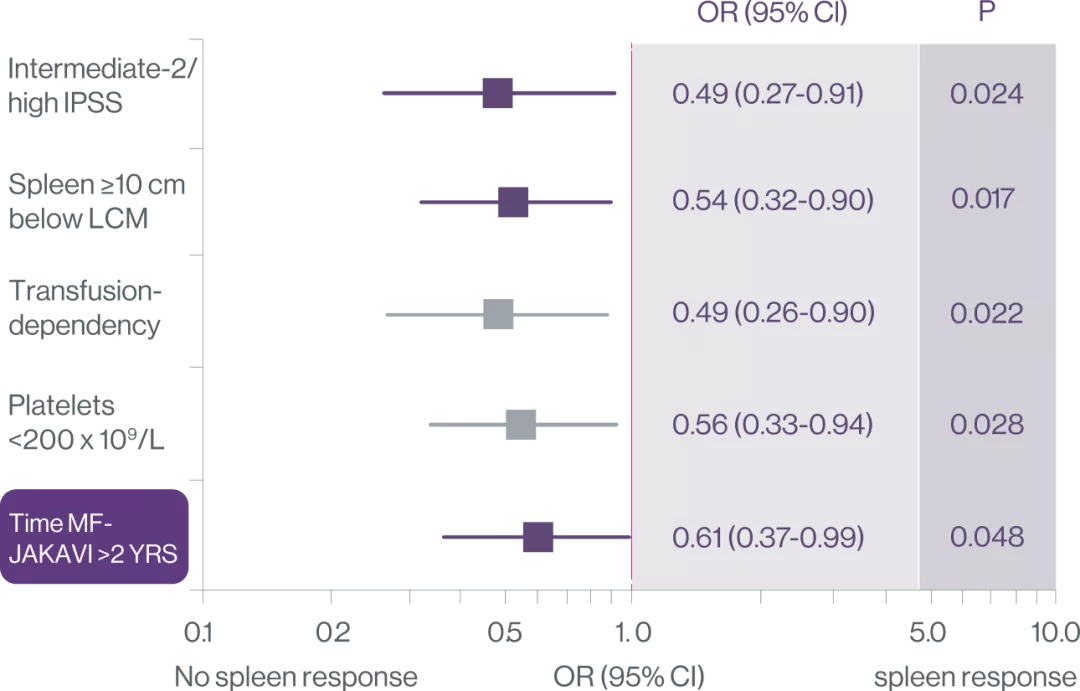
Earlier treatment with JAKAVI increased the probability of spleen response by almost Image

|
For JAKAVI-MF Abbreviated prescribing information
For JAKAVI-MF Abbreviated prescribing information
References
Vannucchi AM, Kantarjian HM, Kiladjian JJ, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100(9):1139-1145.
Verstovsek S, Kantarjian HM, Estrov Z, Cortes JE, Thomas DA, Kadia T, Pierce S, Jabbour E, Borthakur G, Rumi E, Pungolino E. Long-term outcomes of 107 patients with myelofibrosisreceiving JAK1JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood, The Journal of the American Society of Hematology. 2012 Aug 9;120(6):1202-9.
Cervantes F, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Sirulnik A, Stalbovskaya V, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122:4047–53
Palandri F, Palumbo GA, Bonifacio M, et al. Baseline factors associated with response to ruxolitinib: an independent study on 408 patients with myelofibrosis. Oncotarget. 2017;8(45):79073-79086
Approved by Egyptian Drug Authority: HF0424OA4703/102025. Invalidation date: 17/12/2025.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0424OA4703/102025 17/12/2025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
