
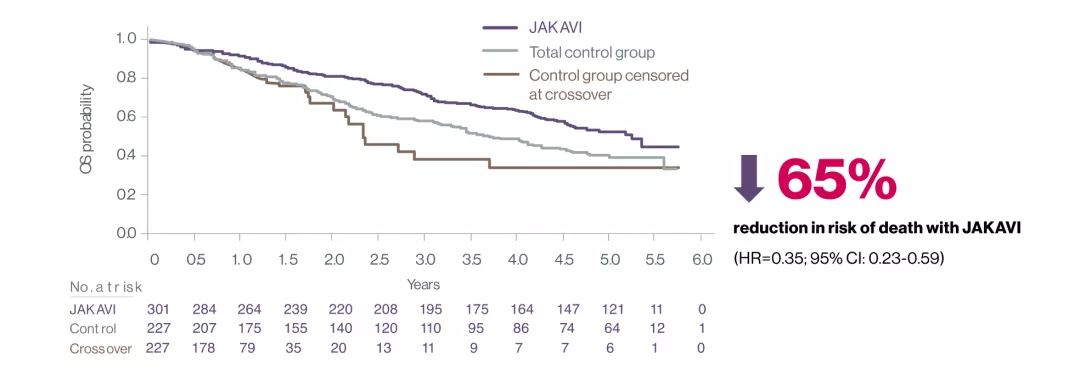
Overall Survival
Treatment with JAKAVI offers your MF patients extended survival benefit1
Myelofibrosis Is Associated With Reduced survival by 40% at 5 years, 60% at 10 years2
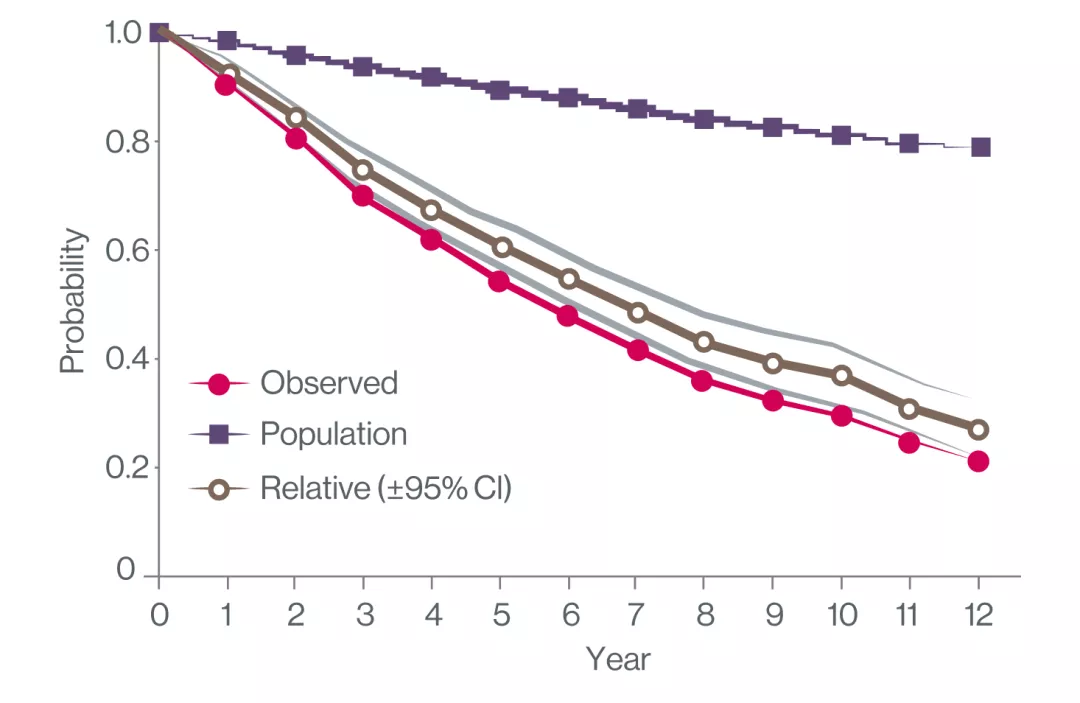
Extend your MF patients’ survival with JAKAVI at diagnosis3
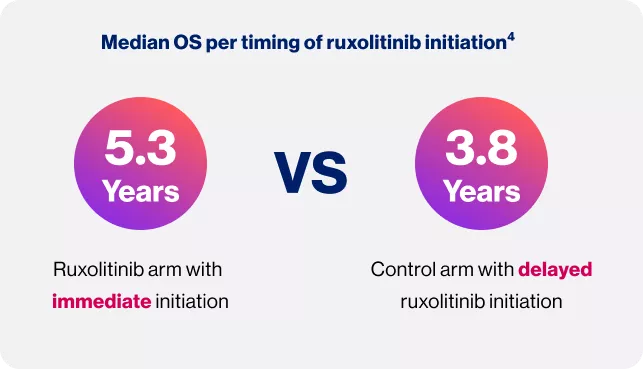
Timing matters: Treat at diagnosis
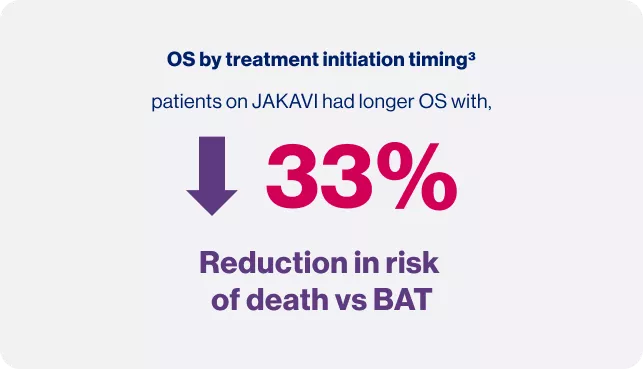
OS: Overall survival
For JAKAVI-MF Abbreviated prescribing information
For JAKAVI-MF Abbreviated prescribing information
References
Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701-1707.
Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, Vannucchi AM, Mesa RA, Demory JL, Barosi G, Rumi E. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood, The Journal of the American Society of Hematology. 2009 Mar 26;113(13):2895-901
Verstovsek S, Gupta V, Gotlib JR, et al. A pooled overall survival (OS) analysis of 5-year data fr om the COMFORT-I and COMFORT-II trials of ruxolitinib for the treatment of myelofibrosis (MF). Blood. 2016;128(22):3110.
Verstovsek S, Gotlib J, Mesa RA, Vannucchi AM, Kiladjian JJ, Cervantes F, Harrison CN, Paquette R, Sun W, Naim A, Langmuir P. Long-term survival in patients
treated with ruxolitinib for myelofibrosis: COMFORT-I and-II pooled analyses. Journal of hematology & oncology. 2017 Dec;10:1-6
Approved by Egyptian Drug Authority: HF0424OA4703/102025. Invalidation date: 17/12/2025.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0424OA4703/102025 17/12/2025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
