
ACEi: angiotensin-converting enzyme inhibitor; CI: confidence interval; CV: cardiovascular; HF: heart failure; HFrEF: heart failure with reduced ejection fraction; HR: hazard ratio; LVEF: left ventricular ejection fraction; OR: odds ratio; KCCQ:Kansas City Cardiomyopathy Questionnaire
* Stronger Heart: In PROVE-HF, LVEF increased vs baseline from 28.2% to 37.8% in patients with HFrEF treated with ENTRESTO® at 12 months (difference, 9.4% [95% CI, 8.8% to 9.9%]; P < .001).1 Stronger Life: In PARADIGM-HF, as compared with an Enalapril, ENTRESTO® reduced the risk of hospitalization for heart failure by 21% (HR, 0.79; 95% CI, 0.71 to 0.89; P<0.001) and change in KCCQ clinical summary score at 8 months (between-group difference, 1.64 points; 95% CI, 0.63 to 2.65; P=0.001).2
† ENTRESTO® must not be administered until 36 hours after discontinuing ACEi therapy.3
‡ HR for HF hospitalization 0.79; 95% CI, 0.71 to 0.89; P<0.0012
§ HR for CV death 0.80; 95% CI, 0.71 to 0.89; P<0.0012
# ENTRESTO® was significantly associated with a 5-point or greater improvement in change score difference in combined physical and social activity mean score with adjustment for baseline score at 8-month follow-up (OR, 1.12; 95% CI, 1.00-1.24; P = .04).4
Meet Our Patients: 3- Newly diagnosed as a HFrEF patient
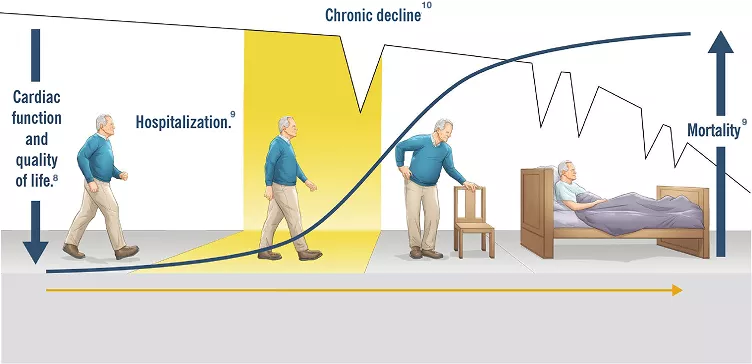
Patients with mild symptoms may still have an increased risk of hospitalization and death.5
Frequent decompensations and hospitalization lead to progressive deterioration of cardiac function and quality of life.6

B.A, 57 Years
Newly diagnosed as a HFrEF patient
He is complaining of shortness of breath which occurs upon slight exertion.
Bakry has a history of CAD.
Echocardiography revealed, dilated left ventricle with left ventricular EF <40%.
not a real patient
Adapted from ref.5
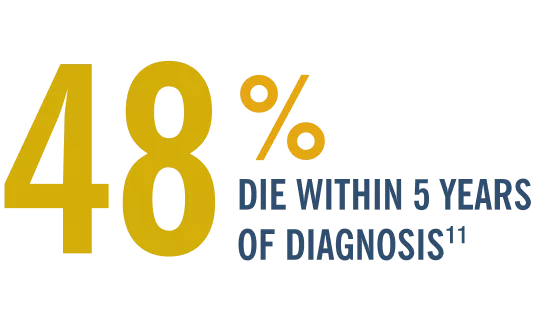
Although approaches to heart failure have improved prognosis, mortality remains high.7
Frequent decompensations and hospitalization lead to progressive deterioration of cardiac function and quality of life.6
A high proportion of deaths in patients with HF occurs suddenly and unexpectedly.12
Sudden cardiac death accounts for approximately half of deaths in patients with HF.12
The proportion of sudden cardiac deaths varies according to NYHA class and is greater in patients with mild-to-moderate symptoms NYHA classes II–III).13,14
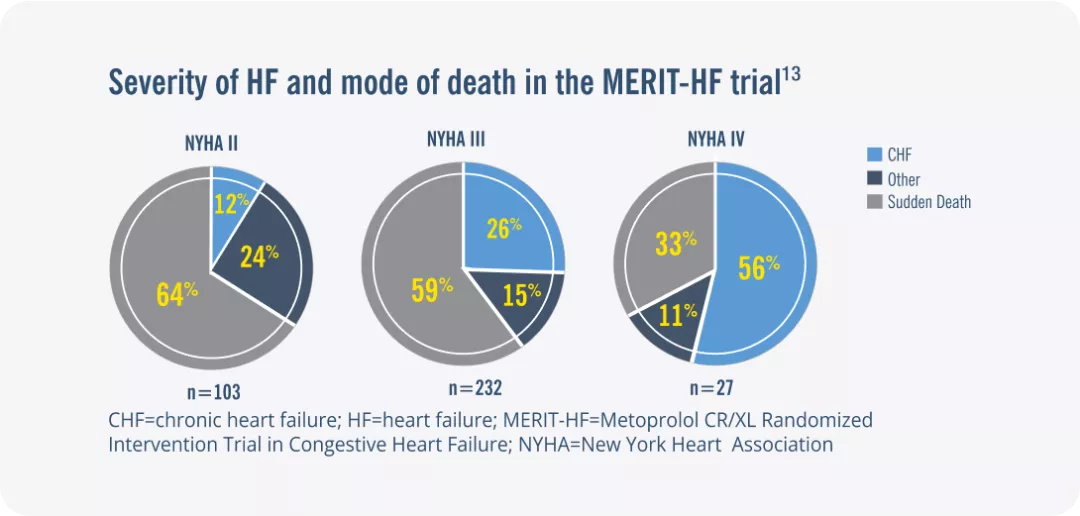
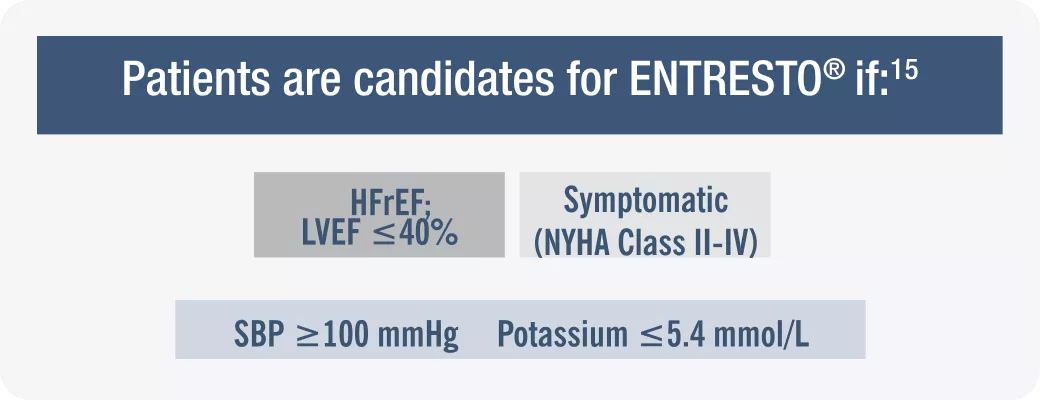
HFrEF= Heart Failure with Reduced Ejection Fraction; ; LVEF=Left ventricular ejection fraction; NYHA=New York Heart. Association
References
Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM. Association of change in N-terminal pro–B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. Jama. 2019 Sep 17;322(11):1085-95.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine. 2014 Sep 11;371(11):993-1004.
Egyptian Drug Authority (EDA). Entresto Approved Leaflet. Approval Date: 07/06/2021.
Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA cardiology. 2018 Jun 1;3(6):498-505.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JG. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European heart journal. 2021 Sep 21;42(36):3599-726.
Mesquita E, Jorge A, Rabelo L, Souza Jr C. Understanding Hospitalization in Patients with Heart Failure. International Journal of Cardiovascular Sciences. 2017; 30 (1): 81-90.
Pons F, Lupón J, Urrutia A, González B, Crespo E, Díez C, Cano L, Cabanes R, Altimir S, Coll R, Pascual T. Mortality and cause of death in patients with heart failure: findings at a specialist multidisciplinary heart failure unit. Revista Española de Cardiología (English Edition). 2010 Mar 1;63(3):303-14.
Holland R, Rechel B, Stepien K, Harvey I, Brooksby I. Patients’ self-assessed functional status in heart failure by New York Heart Association class: a prognostic predictor of hospitalizations, quality of life and death. J Card Fail. 2010; 16 (2): 150-6.
Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006; 151: 444–50.
Gheorghiade M, De Luca L, Fonarow GC, Fillippatos G, Metra M, Francis GC.Pathophysi ologic targets in the early phase of acute heart failure syndromes. Am J Cardiol. 2005; 96 (6A): 11G-17G
Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. Jama. 2004 Jul 21;292(3):344-50.
Authors/Task Force Members, McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European heart journal. 2012 Jul 1;33(14):1787-847.
Merit-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in-congestive heart failure (MERIT-HF). The Lancet.1999 Jun 12;353(9169):2001-7.
Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Rizkala AR, Brahimi A, Claggett B, Finn PV. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. European heart journal. 2015 Aug 7;36(30):1990-7.
Summary Of Product Characteristics (SMPC). Entresto. Available at: https://www.ema.europa.eu/en/documents/product-information/entresto-epar-product-information_en.pdf. Last accessed at: 17/06/2025.
Entresto® API
Entresto® API
Approved by Egyptian Drug Authority: HF0068OA4787/102025. Invalidation date: 04/05/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0068OA4787/102025 04/05/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
