
ACEi: angiotensin-converting enzyme inhibitor; CI: confidence interval; CV: cardiovascular; HF: heart failure; HFrEF: heart failure with reduced ejection fraction; HR: hazard ratio; LVEF: left ventricular ejection fraction; OR: odds ratio; KCCQ:Kansas City Cardiomyopathy Questionnaire
* Stronger Heart: In PROVE-HF, LVEF increased vs baseline from 28.2% to 37.8% in patients with HFrEF treated with ENTRESTO® at 12 months (difference, 9.4% [95% CI, 8.8% to 9.9%]; P < .001).1 Stronger Life: In PARADIGM-HF, as compared with an Enalapril, ENTRESTO® reduced the risk of hospitalization for heart failure by 21% (HR, 0.79; 95% CI, 0.71 to 0.89; P<0.001) and change in KCCQ clinical summary score at 8 months (between-group difference, 1.64 points; 95% CI, 0.63 to 2.65; P=0.001).2
† ENTRESTO® must not be administered until 36 hours after discontinuing ACEi therapy.3
‡ HR for HF hospitalization 0.79; 95% CI, 0.71 to 0.89; P<0.0012
§ HR for CV death 0.80; 95% CI, 0.71 to 0.89; P<0.0012
# ENTRESTO® was significantly associated with a 5-point or greater improvement in change score difference in combined physical and social activity mean score with adjustment for baseline score at 8-month follow-up (OR, 1.12; 95% CI, 1.00-1.24; P = .04).4
Meet our patients: 2- Acute Decompensated Heart Failure
Rates of short-term unplanned rehospitalization and death associated with acute decompensated heart failure are high.5
Patient stabilized during hospitalization for ADHF; The time during the transition to home and throughout the subsequent “vulnerable period,” morbidity and mortality among patients with acute decompensated heart failure remain high.5

N.M, 61 Years
Hospitalized due to HFrEF
Patient is admitted 2 days back and is currently hemodynamically stable.
not a real patient
Adapted from ref.5
HF mortality is high in the first 30 days after discharge.6
Hospital admission for HF is a specific opportunity to optimize HF therapy. Adapted from ref. 6
2x higher mortality risk during first 30 days compared to 6 months after disharge6
1 in 4 patients hospitalized for HF will be readmitted for any cause within 30 days of discharge.7
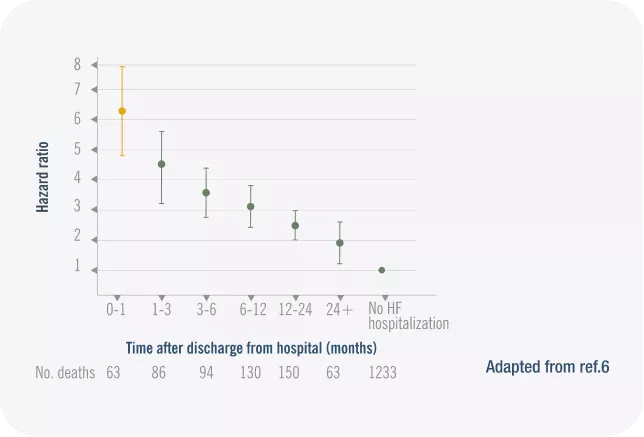

Objective:6
We used data from the CHARM trials to assess the influence of a nonfatal hospitalization for HF on subsequent death in this broad spectrum of HF patients.
Study Design:6
The CHARM program consisted of 3 independent, concurrently performed trials in which 7599 patients with New York Heart\ Association class II to IV chronic HF of 4 weeks’ duration were randomized to candesartan (target dose, 32 mg once daily) or matching placebo added to conventional background treatments.
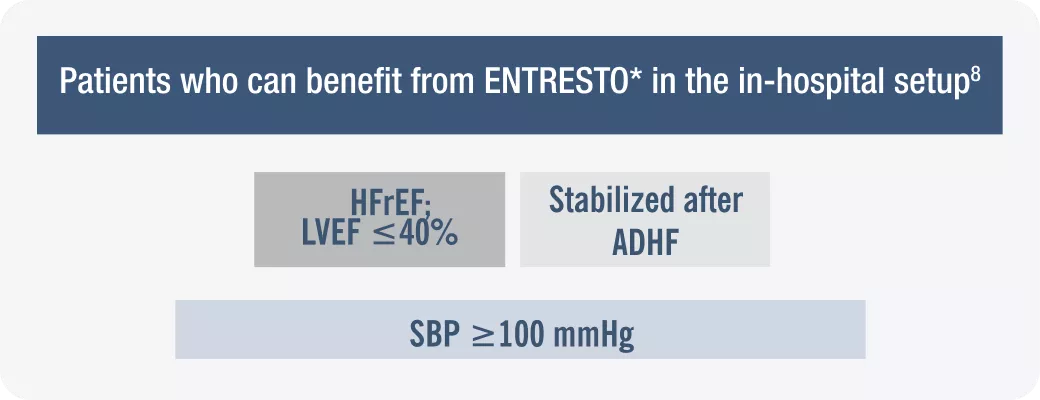
HFrEF= Heart Failure with Reduced Ejection Fraction; ADHF= Acute decompensated heart failure.
References
- Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM. Association of change in N-terminal pro–B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. Jama. 2019 Sep 17;322(11):1085-95.
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine. 2014 Sep 11;371(11):993-1004.
- Egyptian Drug Authority (EDA). Entresto Approved Leaflet. Approval Date: 07/06/2021.
- Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA cardiology. 2018 Jun 1;3(6):498-505.
- Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E. Angiotensin–neprilysin inhibition in acute decompensated heart failure. New England Journal of Medicine. 2019 Feb 7;380(6):539-48.
- Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007 Sep 25;116(13):1482-7.
- Desai AS, Claggett BL, Packer M, Zile MR, Rouleau JL, Swedberg K, Shi V, Lefkowitz M, Starling R, Teerlink J, McMurray JJ. Influence of sacubitril/valsartan (LCZ696) on 30-day readmission after heart failure hospitalization. Journal of the American College of Cardiology. 2016 Jul 19;68(3):241-8.
- Summary Of Product Characteristics (SMPC). Entresto. Available at: https://www.ema.europa.eu/en/documents/product-information/entresto-epar-product-information_en.pdf. Last accessed at: 17/06/2025.
Entresto® API
Entresto® API
Approved by Egyptian Drug Authority: HF0068OA4787/102025. Invalidation date: 04/05/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0068OA4787/102025 04/05/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
