
ACEi: angiotensin-converting enzyme inhibitor; CI: confidence interval; CV: cardiovascular; HF: heart failure; HFrEF: heart failure with reduced ejection fraction; HR: hazard ratio; LVEF: left ventricular ejection fraction; OR: odds ratio; KCCQ:Kansas City Cardiomyopathy Questionnaire
* Stronger Heart: In PROVE-HF, LVEF increased vs baseline from 28.2% to 37.8% in patients with HFrEF treated with ENTRESTO® at 12 months (difference, 9.4% [95% CI, 8.8% to 9.9%]; P < .001).1 Stronger Life: In PARADIGM-HF, as compared with an Enalapril, ENTRESTO® reduced the risk of hospitalization for heart failure by 21% (HR, 0.79; 95% CI, 0.71 to 0.89; P<0.001) and change in KCCQ clinical summary score at 8 months (between-group difference, 1.64 points; 95% CI, 0.63 to 2.65; P=0.001).2
† ENTRESTO® must not be administered until 36 hours after discontinuing ACEi therapy.3
‡ HR for HF hospitalization 0.79; 95% CI, 0.71 to 0.89; P<0.0012
§ HR for CV death 0.80; 95% CI, 0.71 to 0.89; P<0.0012
# ENTRESTO® was significantly associated with a 5-point or greater improvement in change score difference in combined physical and social activity mean score with adjustment for baseline score at 8-month follow-up (OR, 1.12; 95% CI, 1.00-1.24; P = .04).4
Safety Trial:
Entresto® had considerable tolerability. Adapted from ref. 5
With Entresto®, fewer patients stopped their medication because of an adverse event vs enalapril.*2
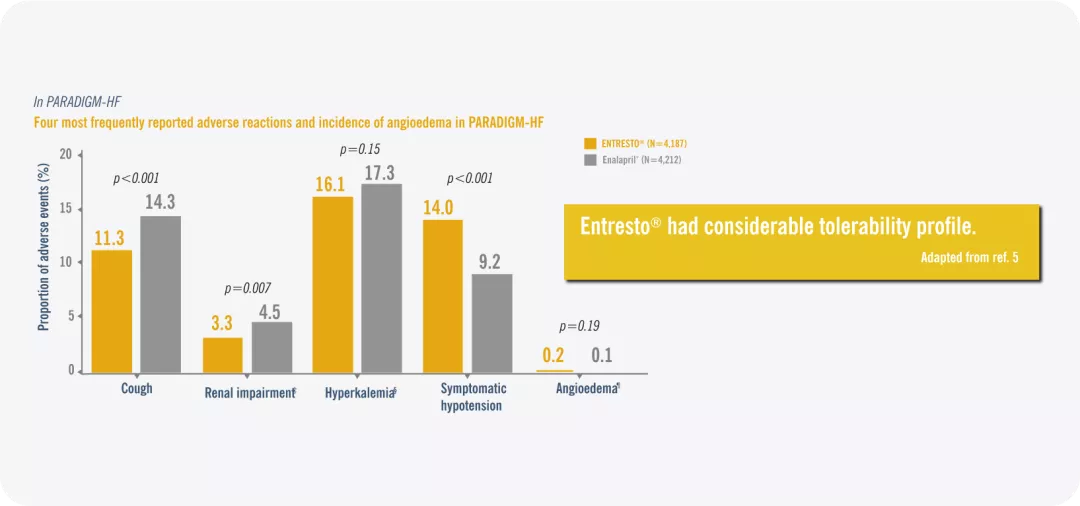
*Enalapril 2X 10 mg daily as comparator vs sacubitril/valsartan 2X 200 mg daily in the PARADIGM-HF (in addition to standard therapy)
‡ Elevated serum creatinine ≥2.5 mg/dL.
§ Elevated serum potassium >5.5 mmol/l.
¶ Angioedema with no treatment or use of antihistamines only
References
Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM. Association of change in N-terminal pro–B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. Jama. 2019 Sep 17;322(11):1085-95.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine. 2014 Sep 11;371(11):993-1004.
Egyptian Drug Authority (EDA). Entresto Approved Leaflet. Approval Date: 07/06/2021.
Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA cardiology. 2018 Jun 1;3(6):498-505.
Senni M, McMurray JJ, Wachter R, McIntyre HF, Reyes A, Majercak I, Andreka P, Shehova‐Yankova N, Anand I, Yilmaz MB, Gogia H. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double‐blind, randomized comparison of two uptitration regimens. European journal of heart failure. 2016 Sep;18(9):1193-202.
Entresto® API
Entresto® API
Approved by Egyptian Drug Authority: HF0068OA4787/102025. Invalidation date: 04/05/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0068OA4787/102025 04/05/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
