
ACEi: angiotensin-converting enzyme inhibitor; CI: confidence interval; CV: cardiovascular; HF: heart failure; HFrEF: heart failure with reduced ejection fraction; HR: hazard ratio; LVEF: left ventricular ejection fraction; OR: odds ratio; KCCQ:Kansas City Cardiomyopathy Questionnaire
* Stronger Heart: In PROVE-HF, LVEF increased vs baseline from 28.2% to 37.8% in patients with HFrEF treated with ENTRESTO® at 12 months (difference, 9.4% [95% CI, 8.8% to 9.9%]; P < .001).1 Stronger Life: In PARADIGM-HF, as compared with an Enalapril, ENTRESTO® reduced the risk of hospitalization for heart failure by 21% (HR, 0.79; 95% CI, 0.71 to 0.89; P<0.001) and change in KCCQ clinical summary score at 8 months (between-group difference, 1.64 points; 95% CI, 0.63 to 2.65; P=0.001).2
† ENTRESTO® must not be administered until 36 hours after discontinuing ACEi therapy.3
‡ HR for HF hospitalization 0.79; 95% CI, 0.71 to 0.89; P<0.0012
§ HR for CV death 0.80; 95% CI, 0.71 to 0.89; P<0.0012
# ENTRESTO® was significantly associated with a 5-point or greater improvement in change score difference in combined physical and social activity mean score with adjustment for baseline score at 8-month follow-up (OR, 1.12; 95% CI, 1.00-1.24; P = .04).4
Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM. Association of change in N-terminal pro–B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. Jama. 2019 Sep 17;322(11):1085-95.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine. 2014 Sep 11;371(11):993-1004.
Egyptian Drug Authority (EDA). Entresto Approved Leaflet. Approval Date: 07/06/2021.
Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA cardiology. 2018 Jun 1;3(6):498-505.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). European journal of heart failure. 2013 Sep;15(9):1062-73.
Summary Of Product Characteristics (SMPC). Entresto. Available at: https://www.ema.europa.eu/en/documents/product-information/entresto-epar-product-information_en.pdf. Last accessed at: 16/06/2025.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiologia Polska (Polish Heart Journal). 2016;74(10):1037-147.
Lewis EF, Claggett BL, McMurray JJ, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD. Health-related quality of life outcomes in PARADIGM-HF. Circulation: Heart Failure. 2017 Aug;10(8):e003430.
Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM. Association of change in N-terminal pro–B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. Jama. 2019 Sep 17;322(11):1085-95.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine. 2014 Sep 11;371(11):993-1004.
Egyptian Drug Authority (EDA). Entresto Approved Leaflet. Approval Date: 07/06/2021.
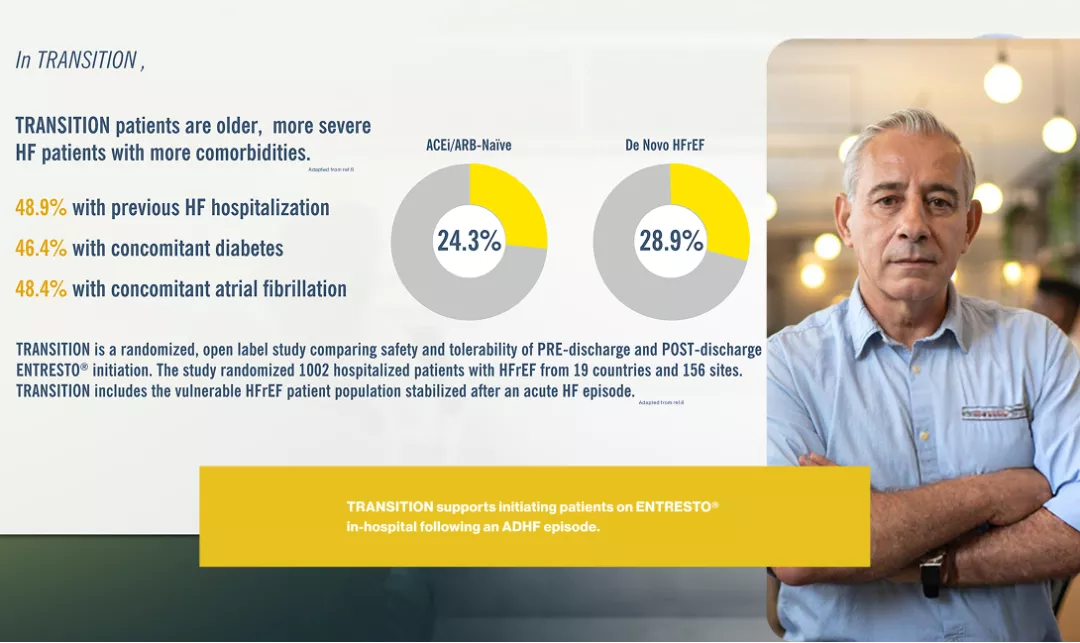
Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA cardiology. 2018 Jun 1;3(6):498-505.Pascual‐Figal D, Wachter R, Senni M, Belohlavek J, Noè A, Carr D, Butylin D. Rationale and design of TRANSITION: a randomized trial of pre‐discharge vs. post‐discharge initiation of sacubitril/valsartan. ESC Heart Failure. 2018 Apr;5(2):327-36.
Wachter R, Senni M, Belohlavek J, Straburzynska‐Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. European journal of heart failure. 2019 Aug;21(8):998-1007.
Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM. Association of change in N-terminal pro–B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. Jama. 2019 Sep 17;322(11):1085-95.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine. 2014 Sep 11;371(11):993-1004.
Egyptian Drug Authority (EDA). Entresto Approved Leaflet. Approval Date: 07/06/2021.
Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA cardiology. 2018 Jun 1;3(6):498-505.
Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E. Angiotensin–neprilysin inhibition in acute decompensated heart failure. New England Journal of Medicine. 2019 Feb 7;380(6):539-48.
Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM. Association of change in N-terminal pro–B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. Jama. 2019 Sep 17;322(11):1085-95.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine. 2014 Sep 11;371(11):993-1004.
Egyptian Drug Authority (EDA). Entresto Approved Leaflet. Approval Date: 07/06/2021.
Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA cardiology. 2018 Jun 1;3(6):498-505.
Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM. Association of change in N-terminal pro–B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. Jama. 2019 Sep 17;322(11):1085-95.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine. 2014 Sep 11;371(11):993-1004.
Egyptian Drug Authority (EDA). Entresto Approved Leaflet. Approval Date: 07/06/2021.
Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA cardiology. 2018 Jun 1;3(6):498-505.
Solomon SD, McMurray JJ, Anand IS, Ge J, Lam CS, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. New England Journal of Medicine. 2019 Oct 24;381(17):1609-20.
Solomon SD, McMurray JJ, Anand IS, Ge J, Lam CS, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. New England Journal of Medicine. 2019 Oct 24;381(17):1609-20. Supplementary Appendix.
PARADIGM-HF is the largest clinical trial ever conducted for heart failure.5
Real Objective:2
To more completely understand the mortality reduction associated with LCZ696, we assessed the effect of LCZ696 compared with enalapril on the mode of death in PARADIGM-HF
Study design:
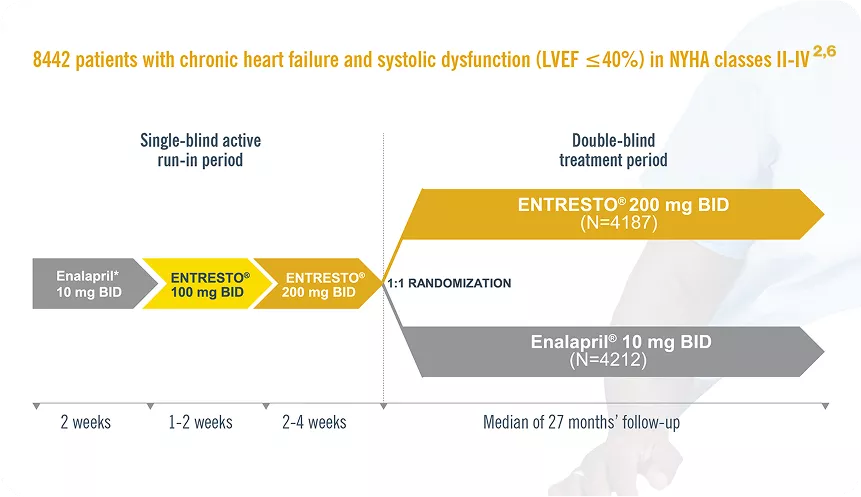
The goals of treatment in patients with HF are to improve quality of life, prevent hospital admission and reduce mortality.7
Entresto® was proven superior to Enalapril in reducing the risks of CV death and of hospitalization for HF.2
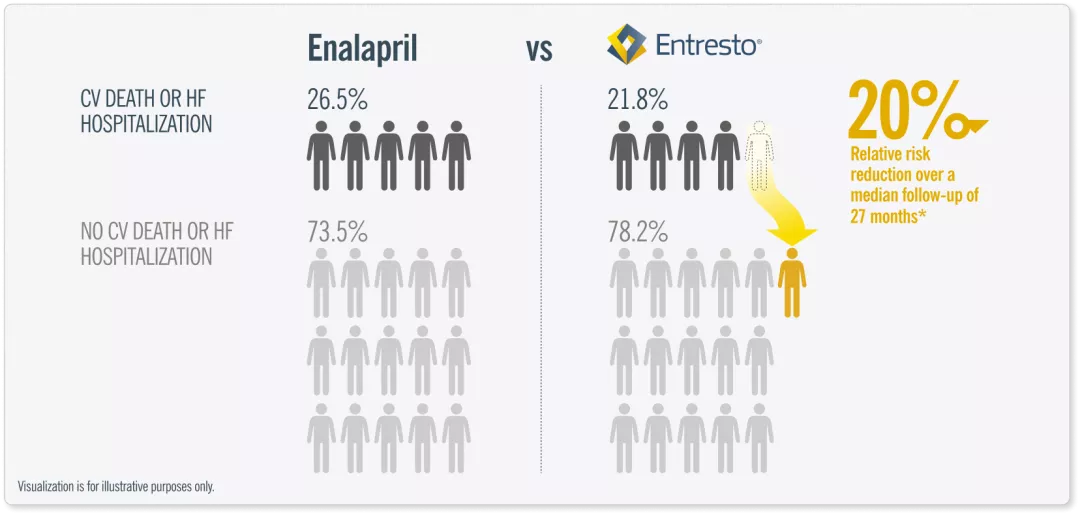
*HR = 0.8 ; 95% CI, 0.73 to 0.87, P < 0.001
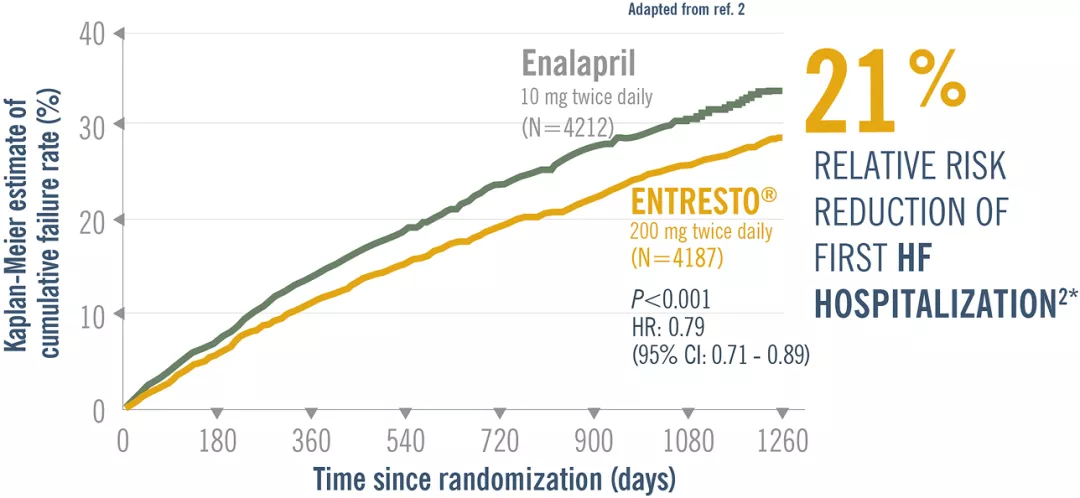
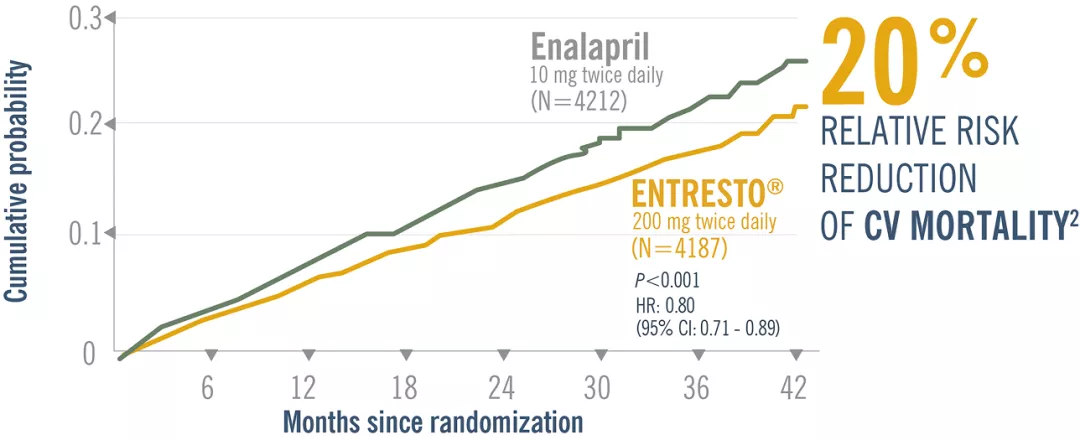
Entresto® reduced the risk of first HF hospitalization by 21% and CV death by 20% vs Enalapril. Adapted from ref 2
Improving quality of life is an important stand-alone target of therapy for heart failure patients.8
In a post hoc analysis, Entresto® improved quality of life Vs. Enalapril. Adapted from ref. 8
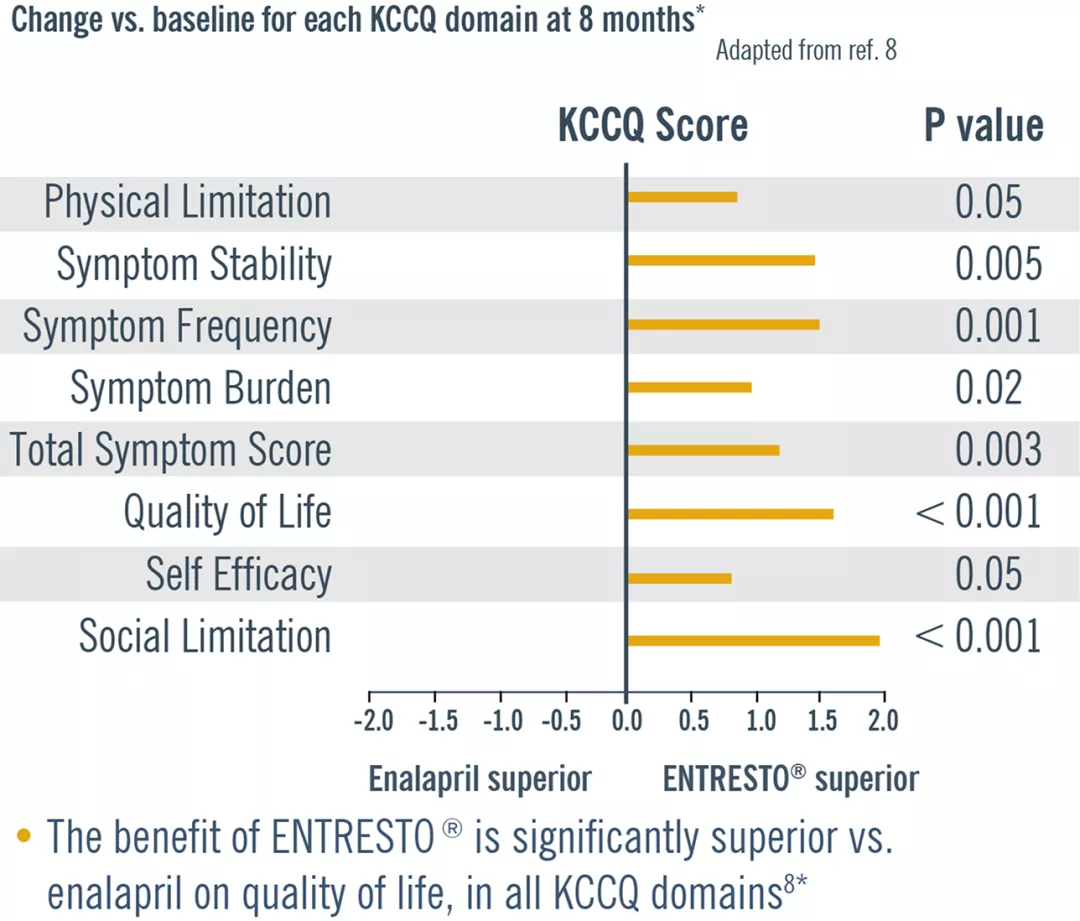
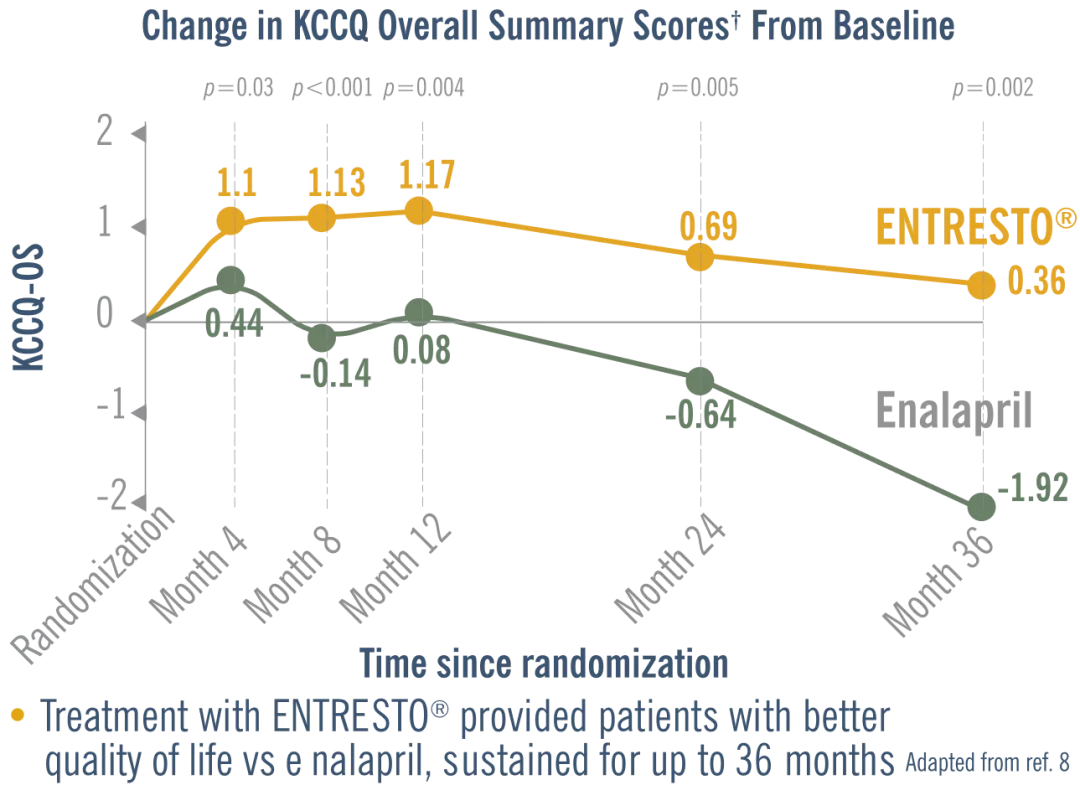
* In surviving patients
Real Objective:8
To determine whether sacubitril/valsartan was superior to enalapril on HRQL changes at 8 months. Secondary objective was to provide an assessment of long term HRQL changes beyond 8 months.
Study Design:8
Eligible patients were entered (in a single blinded fashion) into a run-in phase where they took enalapril 10 mg twice daily for 2 weeks followed by sacubitril/valsartan 100 mg twice daily initially followed by 200 mg twice daily for a 4- to 6-week period. Patients without significant intolerances to either drug were randomized in 1:1 ratio to either enalapril 10 mg twice daily or sacubitril/valsartan 200 mg twice daily in a double-blinded fashion.
BID=twice daily; LVEF=Left ventricular ejection fraction; NYHA=New York Heart Association; ACEi= angiotensin-converting enzyme inhibitor; Cl= confidence interval; CV= cardiovascular; HF= heart failure; HR= hazard ratio. ; KCCQ= Kansas City Cardiomyopathy Questionnaire
References
Efficacy Trials: TRANSITION TRIAL
Real Objective:
The primary objective of the study is to evaluate the proportion of patients in the pre-discharge and post-discharge treatment initiation groups who achieve the target Entresto® dose of 200 mg twice daily at the end of Week 10 after randomization, regardless of any previous temporary dose interruption or down-titration.5
Study design:
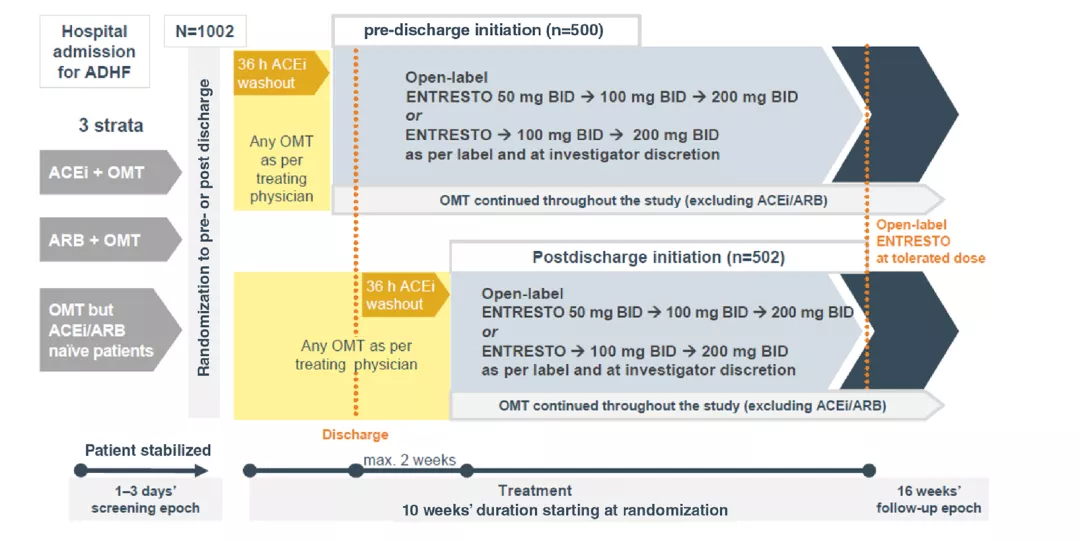
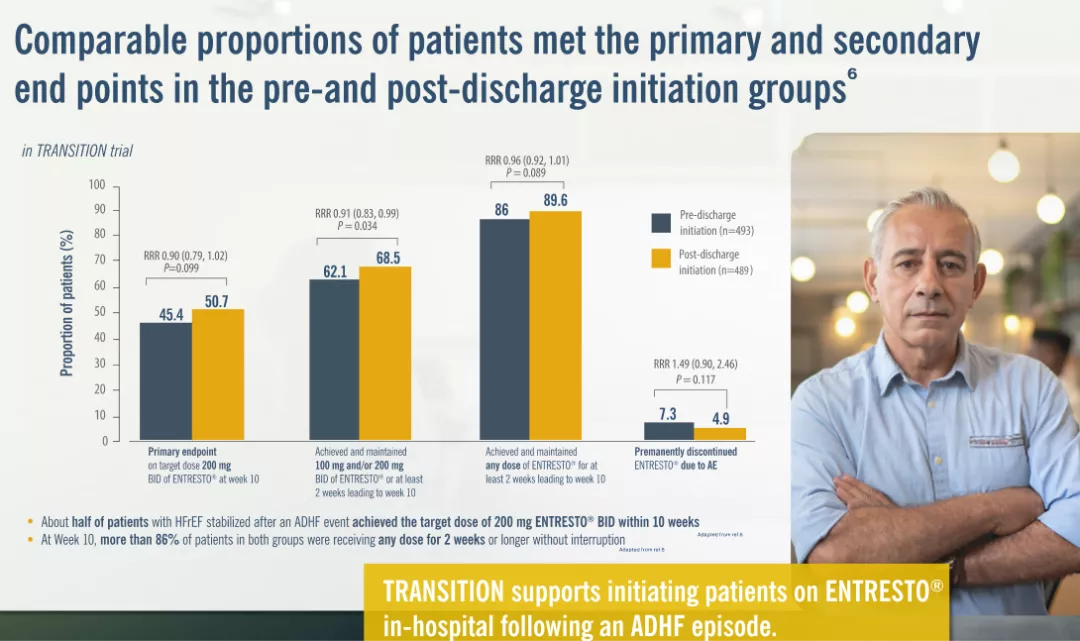
ACEi= Angiotensin converting enzyme inhibitor; ADHE= Acute decompensated heart failure; ARB= Angiotensin Il receptor blocker; BID twice daily; h= hours; HF Heart failure; OMT= Optimised medical therapy.
References
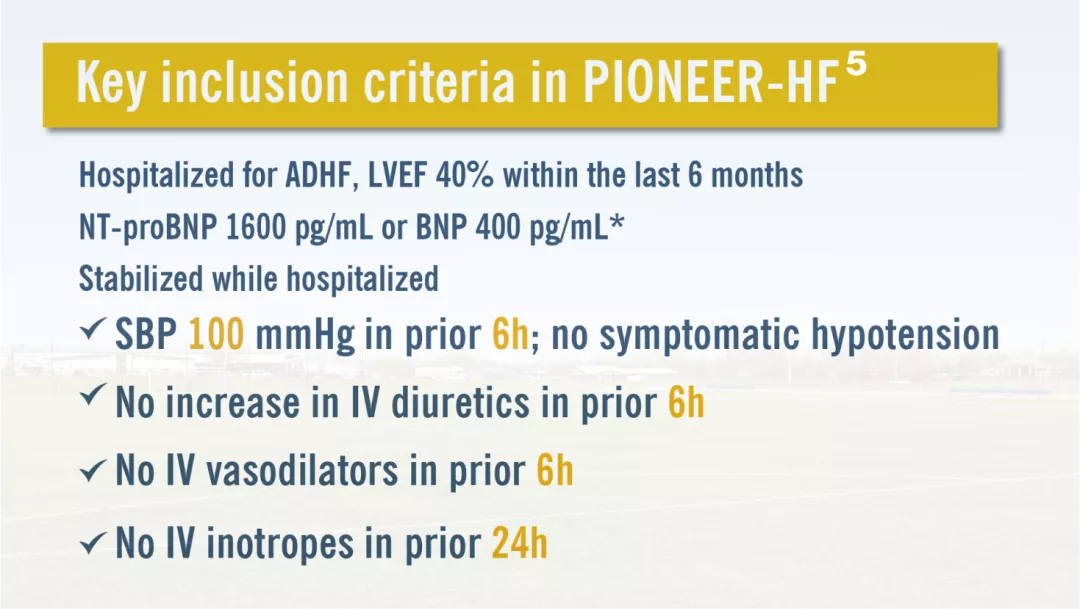
Efficacy Trials: PIONEER-HF TRIAL
Real Objective:5
we designed the PIONEER-HF trial to assess the efficacy and safety of the initiation of sacubitril–valsartan therapy, as compared with enalapril therapy, after hemodynamic stabilization among patients who were hospitalized for acute decompensated heart failure.
Study Design:5
multicenter, randomized, double-blind, active-controlled trial of the in-hospital initiation of sacubitril–valsartan therapy, as compared with enalapril therapy, among patients who had been admitted for acute decompensated heart failure with reduced ejection fraction. During the 8-week trial period, the dose of sacubitril–valsartan was adjusted with a target of 97 mg of sacubitril with 103 mg of valsartan twice daily, and the dose of enalapril was adjusted with a target of 10 mg twice daily.
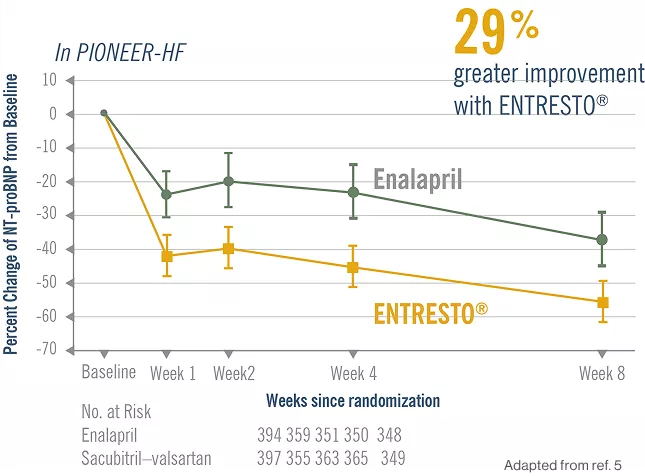
Entresto® has a favorable than Enalapril in-hospital initiation of treatment and continues to be present during the transition to home.5
Initiating Entresto® in-hospital leads to significantly greater and earlier reductions in NT-proBNP vs enalapril, and significantly reduces the risk of serious clinical outcomes after discharge.5
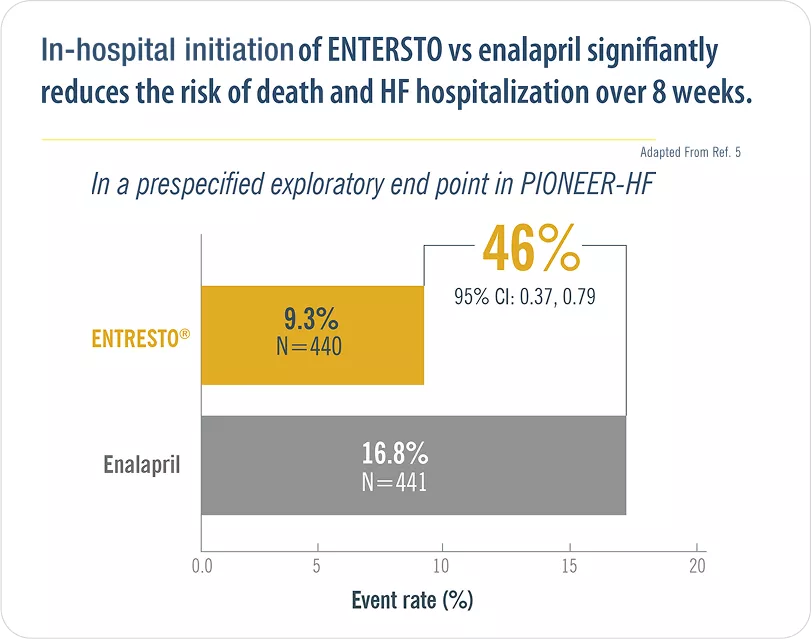
ADHF= Acute decompensated heart failure; BNP= B-type natriuretic peptide; h= hours; IV= Intravenous; LVEF= Left ventricular ejection fraction; NT-proBNP= N-terminal pro–B-type natriuretic peptide; SBP= Systolic blood pressure.
References
Efficacy Trials: PROVE-HF Trial
The correlation between the change in concentration of NT-proBNP and E/e’ was added to the statistical analysis plan prior to the database look.1
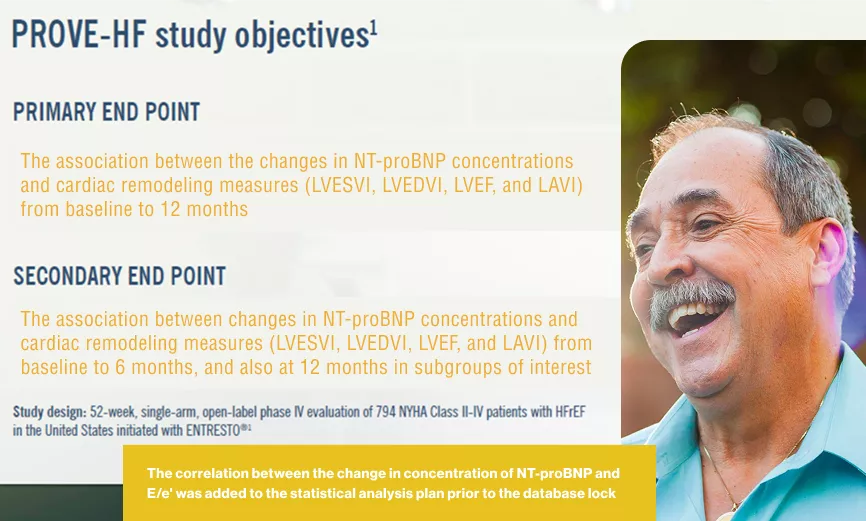
In an exploratory study:
Change in Cardiac Remodeling Measurements from Baseline to 6 and 12 Months After Initiation of Entresto® in All Study Participants1
Entresto® raised median LVEF by 9.4% from baseline in a 12-month exploratory study of patients with HFrEF1*
ENTRESTO® provides rapid and sustained reduction in NT-proBNP as early as 14 days Adapted from ref.1
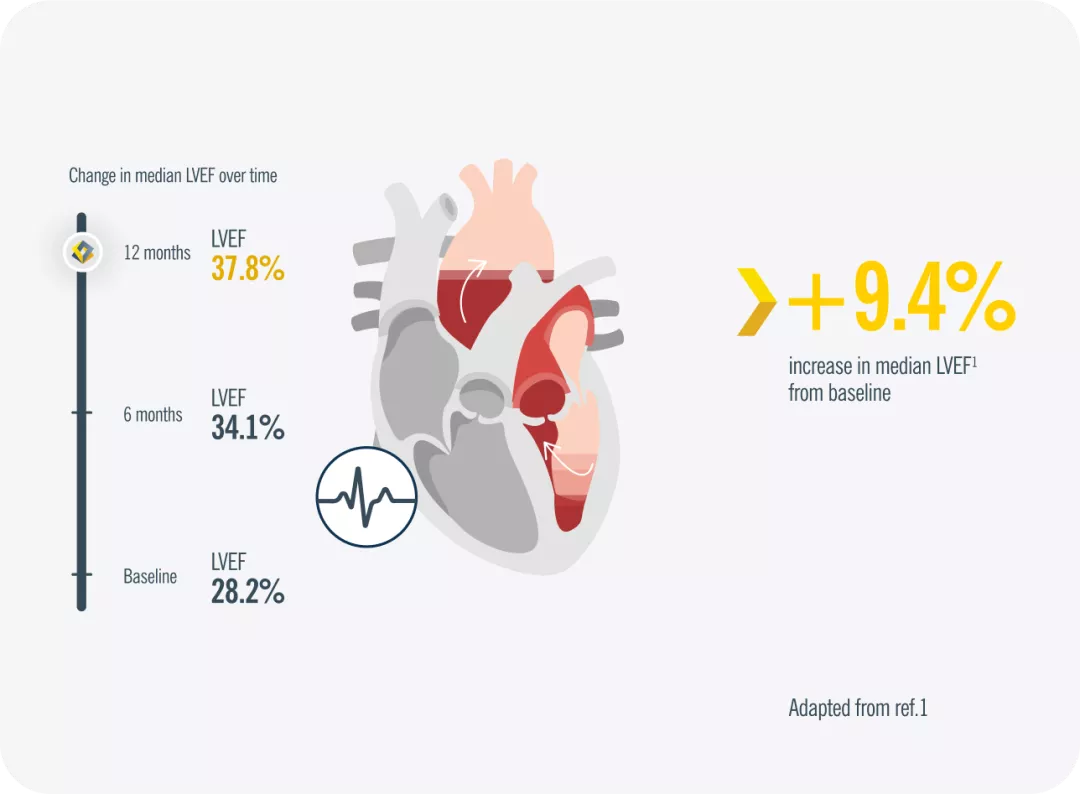
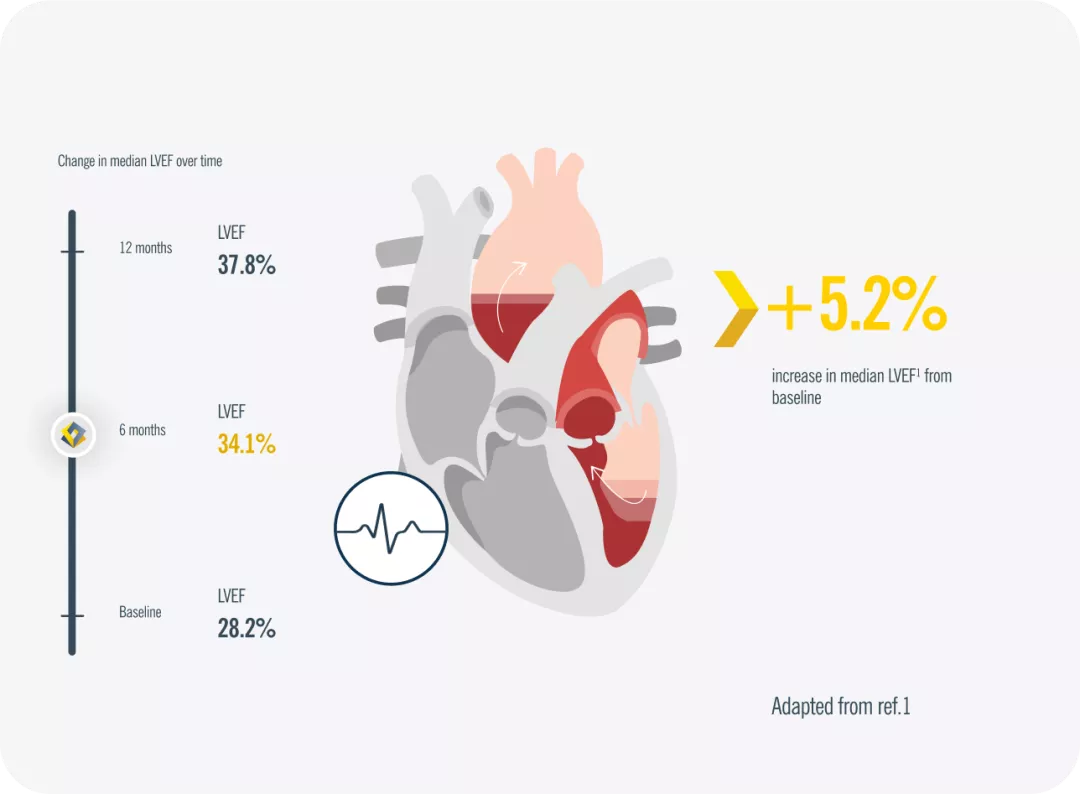
*At 12 months, LVEF increased from 28.2% to 37.8% (difference, 9.4% [95% CI, 8.8% to 9.9%]; P < .001). Includes pre-treated with ACEi/ARB patients and ACEi/ARB-naïve patients1
ACEi= angiotensin-converting enzyme inhibitor; ARB= angiotensin receptor blockers; Cl= confidence interval; HFrEF= heart failure with reduced ejection fraction; LVEF= left ventricular ejection fraction
References
Efficacy Trials: PARAGON-HF Trial
Real Objective:
To test whether ENTRESTO would result in a lower rate of a composite outcome of total hospitalizations for heart failure and death from cardiovascular causes than valsartan.5
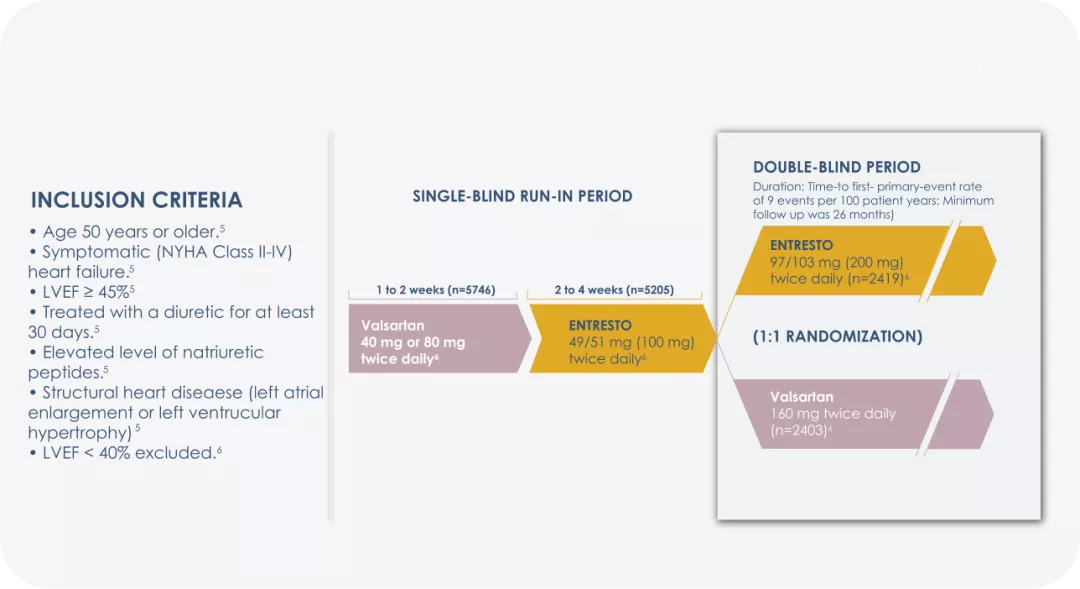
Study design:
ARAGON-HF, was a multicenter, randomized, double-blind trail comparing Entresto® and valsartan in 4822 adult patients with symptomatic heart failure with left ventricular ejection fraction 45%.3
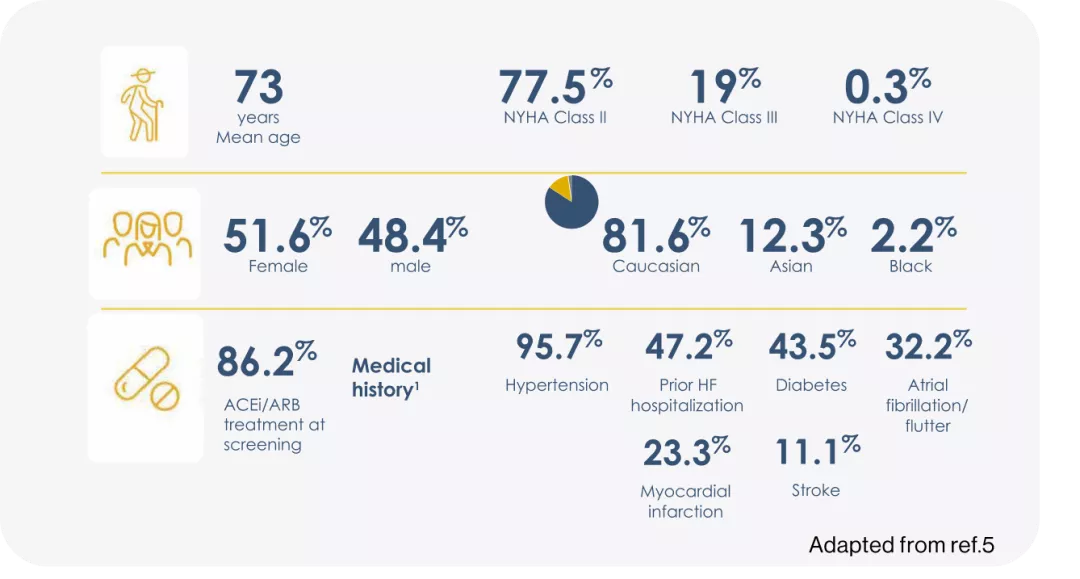
ENTRESTO® had a numerical reduction in the rate of the composite endpoint of total (first and recurrent) HF hospitalizations and CV death vs Valsartan.3
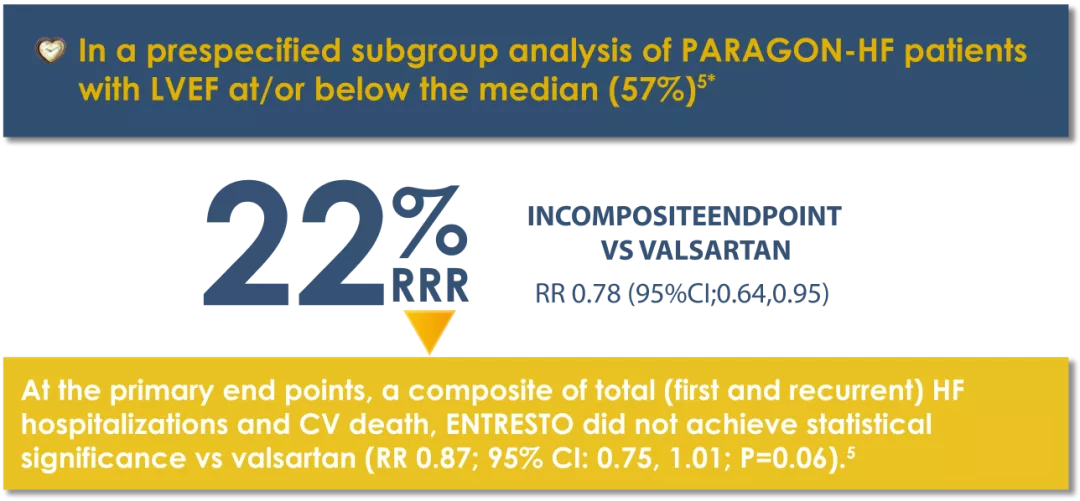
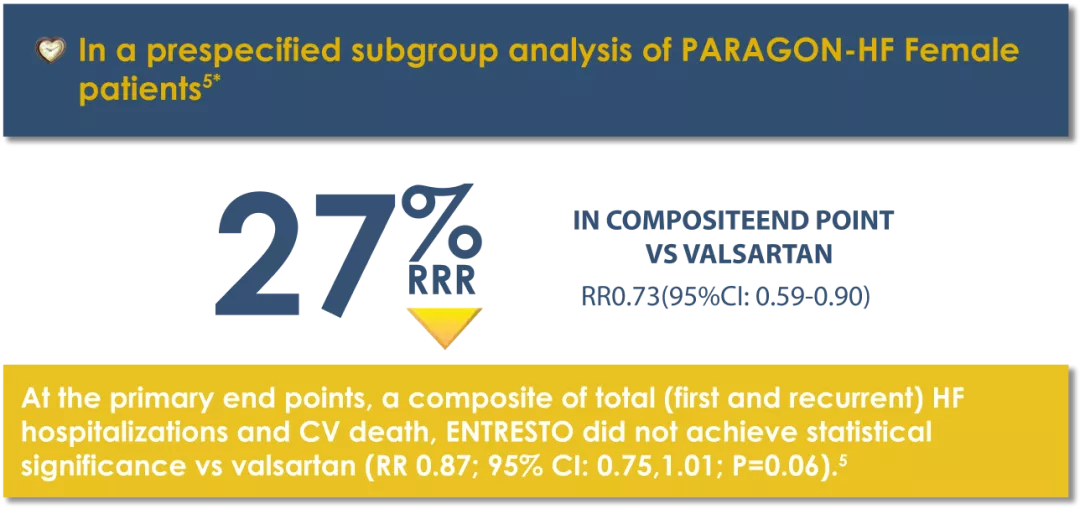
* The patient population of PARAGON-HF met the protocol definition of HFpEF with an LVEF ≥45%, structural heart disease (either LAE or LVH), and no prior echocardiographic LVEF <40%.6
‡Event rate per 100 patient years.

LVEF=Left ventricular ejection fraction; NYHA=New York Heart Association; ACEi= angiotensin-converting enzyme inhibitor; ARB= angiotensin receptor blocker; LAE = Left Atrial Enlargement; LVH = Left Ventricular Hypertrophy.
References
Entresto® API
Entresto® API
Approved by Egyptian Drug Authority: HF0068OA4787/102025. Invalidation date: 04/05/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0068OA4787/102025 04/05/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
