Minimal disease activity (MDA) in psoriatic arthritis (PsA)
Cosentyx® (secukinumab) is indicated for the treatment of: moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adults (alone or in combination with methotrexate [MTX]) who have responded inadequately to disease-modifying anti-rheumatic drug therapy.1
Remission is a poorly defined and variable outcome measure in PsA2,3
PsA is a complex disease comprising six key manifestations: joints, axial, skin, enthesitis, dactylitis and nails.4,5 These are known to cause fatigue, pain and impairment of daily functions, resulting in compromised quality of life.6
Due to its multifaceted clinical presentation, assessment of disease activity can be challenging.5
There is a need for a measure to accurately monitor disease activity across multiple PsA domains to assess holistic disease burden7,8
EULAR and BSR guidelines recommend targeting remission or low disease activity in PsA9,10
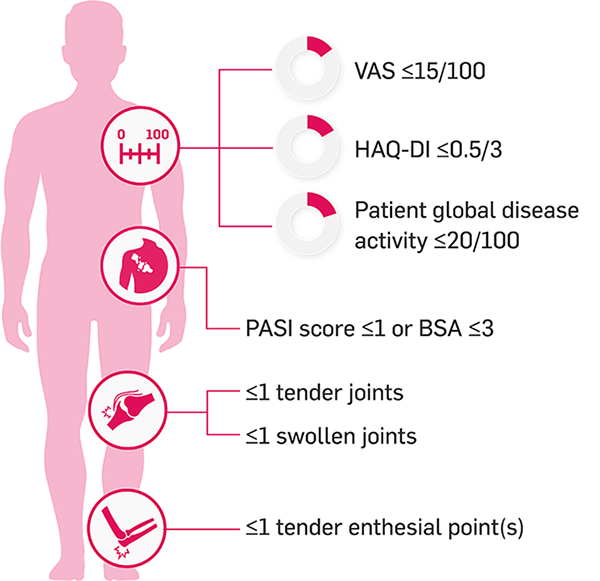
The MDA score is a validated, composite measure that allows the assessment of low disease activity5,9,11
The MDA score is made up of multiple criteria that encompass important aspects of PsA, including joint and skin activity, physical function and pain.8 Patients achieve MDA by meeting 5 of 7 of the following criteria:2,8
What could MDA mean to your eligible patients?
Achieving MDA early is important for long-term outcomes12
Patients who achieved MDA in the first year after their diagnosis demonstrated improved long-term outcomes, including pain, fatigue and emotional wellbeing12
Potential barriers to achieving MDA in patients with PsA

Skin involvement2

High enthesitis count/dactylitis at baseline2

Long-term disease resulting in irreversible damage13

Functional impairment2

Comorbidities such as metabolic syndrome, hepatic steatosis and fibromyalgia2
At 2 years, MDA is more likely to be achieved by those who do not have radiographic progression14

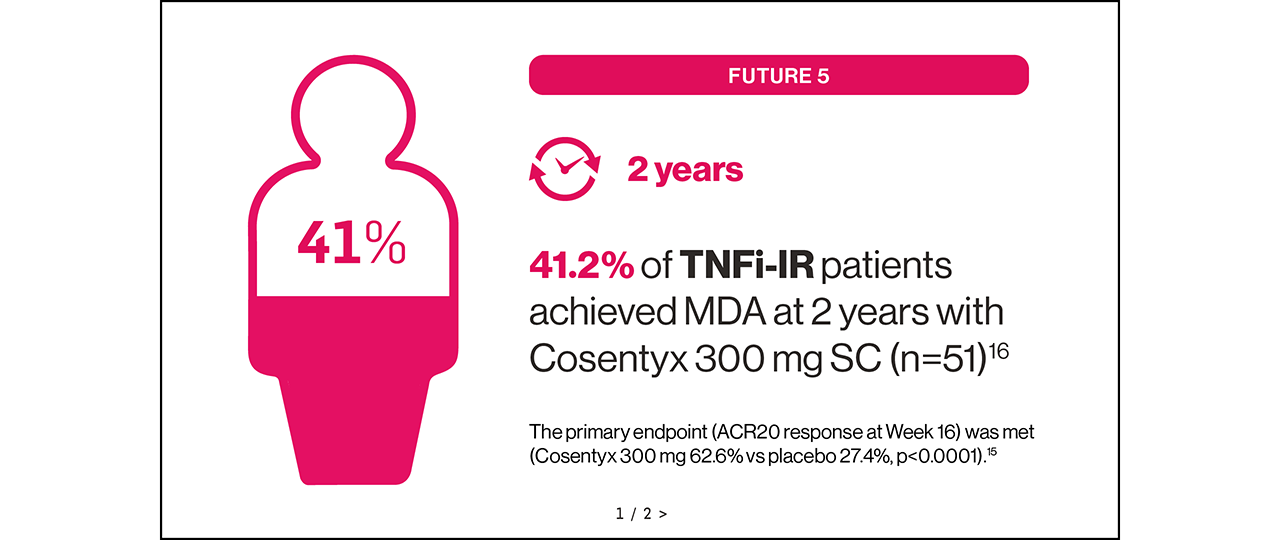
FUTURE 5 trial: The primary endpoint (ACR20 response at Week 16) was met (Cosentyx 300 mg with loading dose 62.6% vs placebo 27.4%, p<0.0001).15
Are you choosing a treatment with MDA in mind?
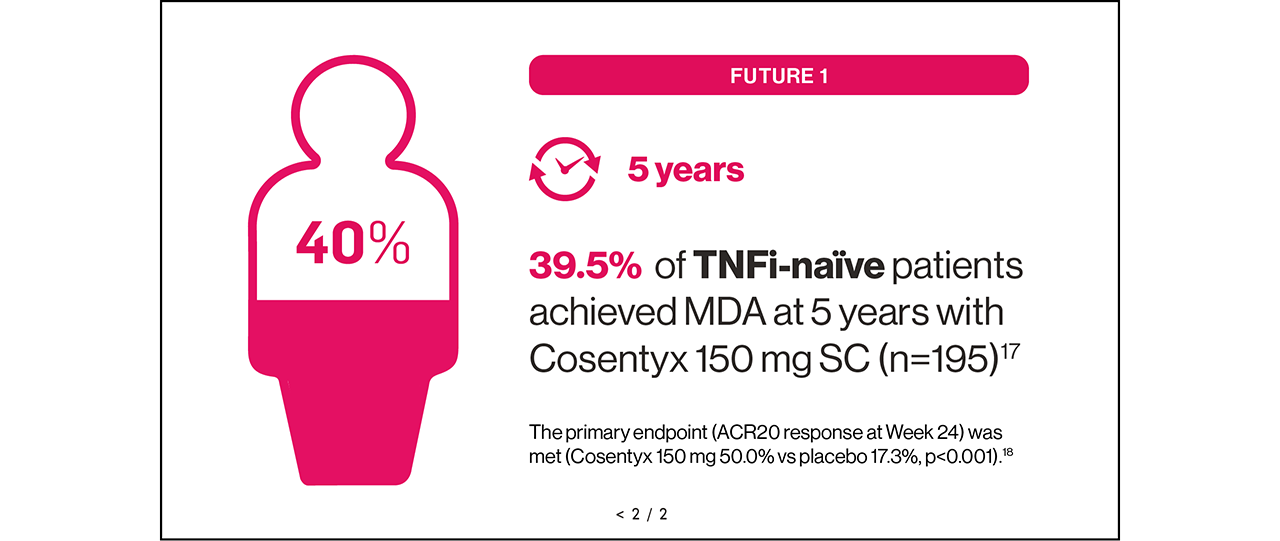
Sustained MDA could be achievable for your eligible adult patients with PsA16,17
SUSTAINED: results up to Year 5.17


Therapeutic Indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
Study designs
FUTURE 5: A randomised, double-blind, Phase III study in patients with active moderate to severe PsA (N=996) randomised to receive Cosentyx 150 mg without loading dose (n=222), Cosentyx 150 mg with loading dose (n=220), Cosentyx 300 mg with loading dose (n=222) or placebo (n=332). The primary endpoint was the percentage of patients with ACR20 response at Week 16.15
FUTURE 1: A multicentre, randomised, double-blind, placebo-controlled, Phase III study in patients with active PsA (N=606) randomised to receive Cosentyx or placebo. Patients in the Cosentyx group received an IV dose of 10 mg/kg of body weight at baseline, Week 2 and Week 4, followed by SC Cosentyx 150 mg or 75 mg every 4 weeks thereafter. The primary endpoint was the percentage of patients with ACR20 response at Week 24.18
Cosentyx is not currently available as an IV treatment.
Please note, Cosentyx should not be used without a loading dose, and the Cosentyx 75 mg dose is not licensed for PsA. The recommended dose for TNFi-IR is 300 mg by SC injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. For other patients, the recommended dose is 150 mg by SC injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, this can be increased to 300 mg.1
Cosentyx has not been studied in renal/hepatically impaired patient populations. No dose recommendations can be made.1
For patients with concomitant moderate to severe PsO, please refer to adult PsO recommendations in the Cosentyx SmPC.1
ACR, American College of Rheumatology; AS, ankylosing spondylitis; BSA, body surface area; BSR, British Society for Rheumatology; ERA, enthesitis-related arthritis; EULAR, European Alliance of Associations for Rheumatology; HAQ-DI, health assessment questionnaire-disability index; HS, hidradenitis suppurativa; IR, inadequate responder; IV, intravenous; JPsA, juvenile psoriatic arthritis; MDA, minimal disease activity; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; PASI, psoriasis area and severity index; PsA, psoriatic arthritis; PsO, plaque psoriasis; SC, subcutaneous; SmPC, summary of product characteristics; TNFi, tumour necrosis factor inhibitor; VAS, visual analogue scale.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Gossec L et al. J Rheumatol 2018;45(1):6–13.
Lubrano E, et al. Clin Exp Rheumatol 2018;36(5):900–910.
Baraliakos X, et al. Ann Rheum Dis 2021;80(5):582–590.
McGagh D & Coates LC. Rheumatology 2020;59(Suppl 1):i29–i36.
Skougaard M, et al. Rheum Adv Pract 2021;5(3):rkab076.
Hackett S & Coates LC. Musculoskeletal Care 2022;20(Suppl 1):S22–S31.
Coates LC, et al. Ann Rheum Dis 2010;69(1):48–53.
Gossec L, et al. Ann Rheum Dis 2020;79(6):700–712.
Tucker L, et al. Rheumatology 2022;61(9):e255–e266.
Scriffignano S, et al. Abstract AB1135. Ann Rheum Dis 2023;82(Suppl 1):1798.
Henkemans SVJS, et al. RMD Open 2022;8(2):e002706.
Mease PJ, et al. RMD Open 2017;3(1):e000415.
Mease PJ, et al. Abstract POS0972. Ann Rheum Dis 2024;83(Suppl1):682–683.
Mease PJ, et al. Ann Rheum Dis 2018;77(6):890–897.
Novartis Data on File. CAIN457F2342 (FUTURE 5): Week 104 Interim Report. May 2019.
Mease PJ, et al. ACR Open Rheumatol 2020;2(1):18–25.
Mease PJ, et al. N Engl J Med 2015;373(14):1329–1339.
UK | July 2025 | FA-11426990
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.