Plaque psoriasis (PsO) patient impact
Cosentyx® (secukinumab) is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy.1
A ‘getting it right first time’ approach is vital with biologics in PsO for optimal management*
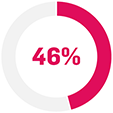
Treatment failure with TNF inhibitors is common over time.2–4 Up to 50 out of every 100 who start TNFi for PsO or psoriatic disease stop within 3 years of treatment initiation due to lack or loss of effectiveness.2–4
Response rates may diminish with every cycle of biologic therapy5–7
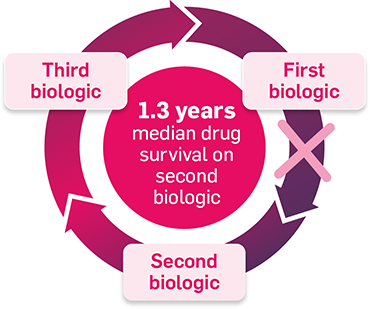
Adapted from Glintborg B, et al. 2013.5
Switching to a second TNFi may not provide the same control as the first5,7
In patients with PsO who switched to a second TNFi (data from an Italian prospective registry study between September 2005– September 2010; N=5423):8
Results were similar in patients who did not switch TNFi: PASI 75: 42.5% at Week 24; p=0.090.8
In patients with psoriatic disease (data from an observational Danish registry study; N=1422):5
In this observational cohort study based on data from the DANBIO registry, patients who were diagnosed with PsA were included. 1,422 patients were included.5
How could a ‘getting it right first time’ approach help improve outcomes and save time for you and your patients?
SIGNATURE trial
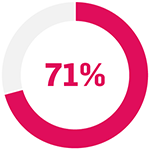
In patients with PsO:9
with inadequate response to 1 TNFi (n=14)

with initial response to 1 TNFi, subsequent LOR (n=44)
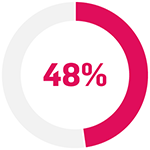
with inadequate response to ≥2 TNFis (n=44)
... achieved PASI75 with Cosentyx 300 mg at Week 169
p<0.0001 compared with baseline for all sub-groups.
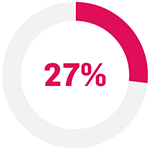
The primary endpoint (PASI 75 response at Week 16) was met (Cosentyx 300 mg 65% vs baseline; p<0.0001).9
In the FUTURE 2 study
Results sustained through to Week 26011
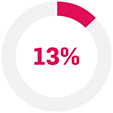
31% (5/16) of TNFi non-responder patients achieved ACR50 with Cosentyx 300 mg at Week 260 (data as observed).11
The primary endpoint (ACR20 response at Week 24) was met (Cosentyx 300 mg: 54% vs placebo: 15%; N=397; p<0·0001).10
Dermatologists can support optimal service and care delivery through:
Discover why overall management of psoriatic disease requires a holistic management approach
Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe PsO in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active PsA in adult patients (alone or in combination with MTX) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active AS in adults who have responded inadequately to conventional therapy; active nr-axSpA with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe HS (acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active ERA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active JPsA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
Study designs
SIGNATURE: 72-week study in 235 patients ≥18 years with moderate-to-severe chronic PsO (PASI ≥10 and DLQI >10). Patients had failed to achieve the required efficacy response to prior anti-TNF therapy according to the NICE criteria for inadequate responders. Any patients whose dose was increased from 150 mg to 300 mg at either Week 16 (end of initiation period) or Week 48 (end of maintenance period 1) were excluded from any efficacy analyses presented here. Only patients who started and stayed on the same dose were considered for the purpose of efficacy assessments.9
The recommended dose is 300 mg of Cosentyx by SC injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for patients with a body weight of ≥90kg.1
FUTURE 2: Phase III, double-blind, placebo-controlled study in which adults with active PsA were randomised to receive SC placebo or Cosentyx 300 mg and 150 mg weekly from baseline, and then every 4 weeks from Week 4. The primary endpoint was the proportion of patients achieving a ≥20% improvement in ACR20 at Week 24.11
In anti-TNFα inadequate responders patients, 150 mg is off-label. The licensed dose is 300 mg Cosentyx with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1
Cosentyx 75 mg is not a licenced dose for PsA.1
*The Getting it Right First Time (GIRFT) programme is a national NHS programme designed to improve the treatment and care of patients.17
ACR, American College of Rheumatology; AS, ankylosing spondylitis; DLQI, dermatology life quality index; ERA, enthesitis-related arthritis; GIRFT, Getting it Right First Time; HS, hidradenitis suppurativa; JPsA, juvenile psoriatic arthritis; LOR, loss of response; MTX, methotrexate; NICE, National Institute of Health and Care Excellence; nr-axSpA, non-radiographic axial spondyloarthritis; NNT, number needed to treat; PASI, psoriasis area and severity index; PsA, psoriatic arthritis; PsO, plaque psoriasis; SC, subcutaneous; TNFi, tumour necrosis factor inhibitor.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Lin P-T, et al. Sci Rep 2018;8(1):16068.
Pina Vegas L, et al. JAMA Dermatol 2022;158(5):513–522.
Rusinol L, et al. Expert Review of Clinical Immunology. 2024;20(1):71–82.
Glintborg B, et al. Arthritis Rheumatol 2013;65(5):1213–1223.
Iskandar, et al. J Invest Dermatol 2018;138(4):775–784.
Gollins, et al. Skin Health Dis 2024;4(2):e350.
Piaserico, S, et al. J Am Acad Dermatol 2014;70(2):257–262.
Warren RB, et al. Br J Dermatol 2020;183(1):60–70.
McInnes IB,, et al. Lancet 2015;386(9999):1137–1146.
McInnes IB, et al. Lancet Rheumatol 2020;2(4):e227–e235.
Wong JLC, et al. Future Hospital J 2017;4(1):23–26.
Wilson FC, et al. Arthritis Rheum 2009;61(2):233–239.
Coates LC, et al. Nat Rev Rheumatol 2022;18(8):465–479.
Tucker ,L et al. Rheumatology (Oxford) 2022;61(9):e255–e266.
Smith CH, et al. Br J Dermatol 2020;183(4):628–637.
NHS England. Getting It Right First Time (GIRFT). Available from: https://gettingitrightfirsttime.co.uk/ [last accessed: June 2025].
UK | June 2025 | FA-11433165
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.