

Cosentyx® (secukinumab): Safety profile and side effects
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
Please refer to the Summary of Product Characteristics (SmPC) for further information. The UK SmPC can be found here.
Cosentyx has a consistent safety profile across indications, demonstrated by over a decade of real-world clinical experience*1,2
*Since first indication in 2015 for eligible adults with moderate to severe plaque psoriasis.2
1 million+ patient-years of exposure globally, across indications3

No discontinuations in pooled clinical trial data and RWE due to Candida infections, with all being non-serious and mild to moderate in severity, except four cases in the PsO pool that were considered severe4

No trend towards increased rates of major adverse cardiovascular event (MACE), malignancy or inflammatory bowel disease (IBD) reported in clinical trials or RWE5

In paediatric patients aged 6 years and over with JIA, Cosentyx demonstrated a safety profile consistent with adult indications1
Please read the full warnings and precautions (found in the SmPC) when prescribing Cosentyx.
RWE shows a consistent safety profile up to 9 years in adult indications5
No trend towards increased adverse event (AE) rates over time (pooled data; AS, PsA and PsO in a PSUR including exposure in clinical trials and marketing experience).5
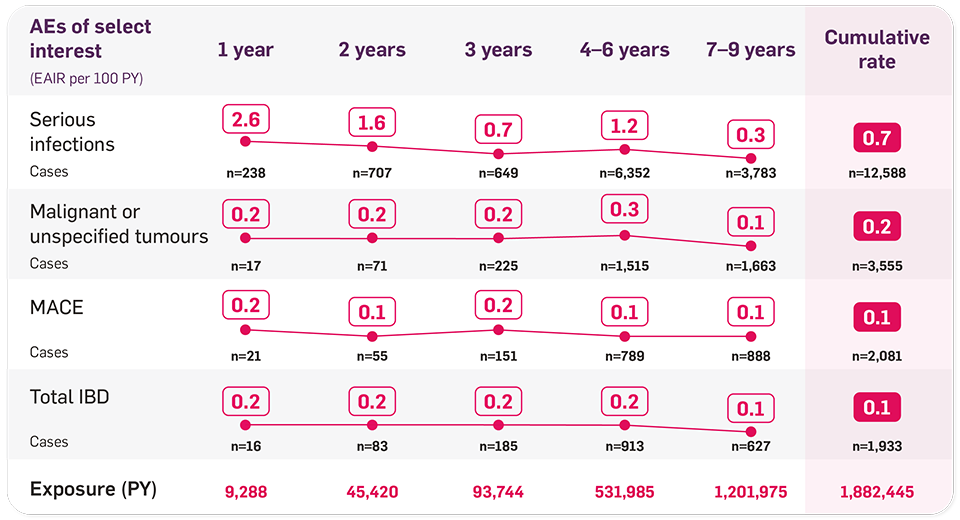
Source: Novartis Data on File, 2025.5
Successive time periods of PSUR shown with cumulative rate: 26 Dec 2014 to 25 June 2015; 26 June 2015 to 25 Dec 2015; 26 Dec 2015 to 25 June 2016; 26 June 2016 to 25 Dec 2016; 26 Dec 2016 to 25 Dec 2017; 26 Dec 2017 to 25 Dec 2018; 26 Dec 2018 to 25 Dec 2019; 26 Dec 2019 to 25 Dec 2020; 26 Dec 2020 to 25 Dec 2023.5
Cosentyx is not recommended in patients with IBD. Please refer to the SmPC for special warnings and precautions for use.1
No trend toward increased rates of malignancy, MACE, or IBD over time5
Summary of AEs from clinical trials and post-marketing experience1
The most frequently reported AEs were upper respiratory tract infections (17.1%; most frequently nasopharyngitis, rhinitis). The safety profile is consistent across indications.1
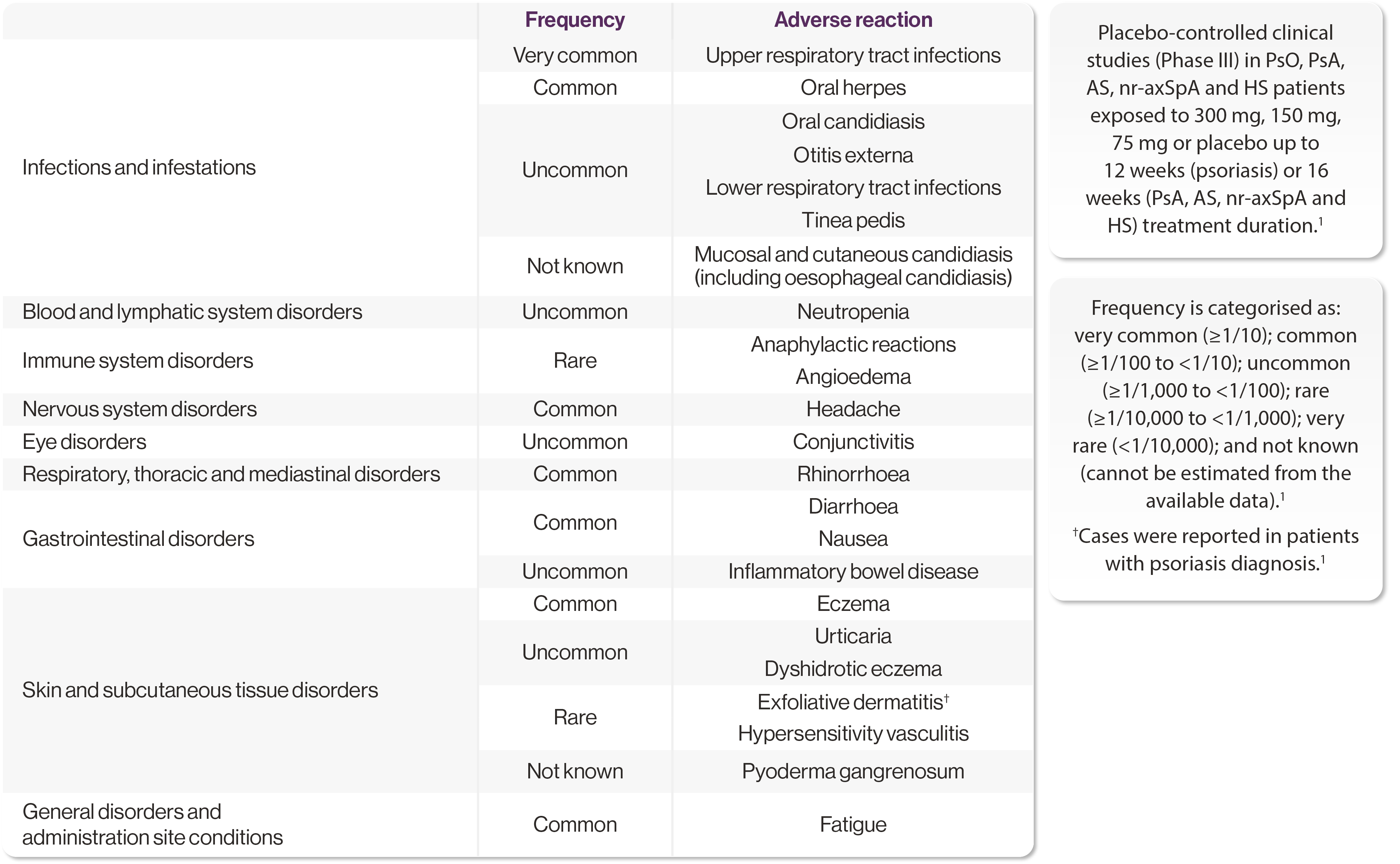
Adapted from Cosentyx SmPC.1
Please refer to the SmPC for full prescribing information and safety profile.1
Safety considerations and precautions1
Please refer to the SmPC for detailed safety profile data and full prescribing and administration information, including dosing in special populations and warnings/precautions.1
Cosentyx has not been studied in patients with renal or hepatic impairment. No dose recommendations can be made.1
Cosentyx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which Cosentyx is indicated. Please refer to the Cosentyx SmPC for full product information before prescribing.1
Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe PsO in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active PsA in adult patients (alone or in combination with MTX) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active AS in adults who have responded inadequately to conventional therapy; active nr-axSpA with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe HS (acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active ERA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active JPsA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
AE, adverse event; AS, ankylosing spondylitis; EAIR, exposure-adjusted incidence rate; ERA, enthesitis-related arthritis; HBV, hepatitis B virus; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; JIA, juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis; MACE, major adverse cardiovascular event; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; PsO, plaque psoriasis; PSUR, periodic safety update report; PY, patient-years; RWE, real-world evidence; SmPC, summary of product characteristics.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
European Medicines Agency. Summary of positive opinion EMA/CHMP/670/0627/2015. Available at: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-cosentyx_en.pdf [Accessed June 2025].
Novartis Data on File. Secukinumab (SEC018). February 2025.
Deodhar A, et al. Arthritis Res Ther 2019;21(1):111.
Novartis Data on File. Secukinumab (SEC020). April 2025.
Schreiber S, et al. Ann Rheum Dis 2019;78(4):473–479.
UK | June 2025 | FA-11328448-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.










