Resources for HCPs
Discover tools to support the use of ILARIS in day-to-day practice.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Periodic fever syndromes
ILARIS® (canakinumab) is indicated for the treatment of the following autoinflammatory periodic fever syndromes in adults, adolescents and children aged 2 years and older:
Cryopyrin-associated periodic syndromes (CAPS), including:
Muckle-Wells syndrome (MWS)
Neonatal-onset multisystem inflammatory disease (NOMID)/chronic infantile neurological, cutaneous, articular syndrome (CINCA)
Severe forms of familial cold autoinflammatory syndrome (FCAS)/familial cold urticaria (FCU) presenting with signs and symptoms beyond cold-induced urticarial skin rash
Tumour necrosis factor receptor associated periodic syndrome (TRAPS)
Hyperimmunoglobulin D syndrome (HIDS)/mevalonate kinase deficiency (MKD)
Familial Mediterranean Fever (FMF)
ILARIS should be given in combination with colchicine, if appropriate
Still’s disease
ILARIS is indicated in patients who have responded inadequately to previous therapy with non-steroidal anti-inflammatory drugs (NSAIDs) and systemic corticosteroids, for the treatment of active Still’s disease, including:
Adult-onset Still’s disease (AOSD)
Systemic juvenile idiopathic arthritis (SJIA) in patients aged 2 years and older
ILARIS can be given as monotherapy or in combination with methotrexate
Gouty arthritis
ILARIS is indicated for the symptomatic treatment of adult patients with frequent gouty arthritis attacks (at least 3 attacks in the previous 12 months) in whom NSAIDs and colchicine are contraindicated, are not tolerated, or do not provide an adequate response, and in whom repeated courses of corticosteroids are not appropriate.
Resources for HCPs to share with patients who have been prescribed ILARIS to treat their periodic fever syndrome or Still’s disease.
Please do not show this webpage to patients, or direct them to this webpage. Instead, please direct patients to the patient portal.
A booklet about what ILARIS is, how it works, and things to know before starting treatment.
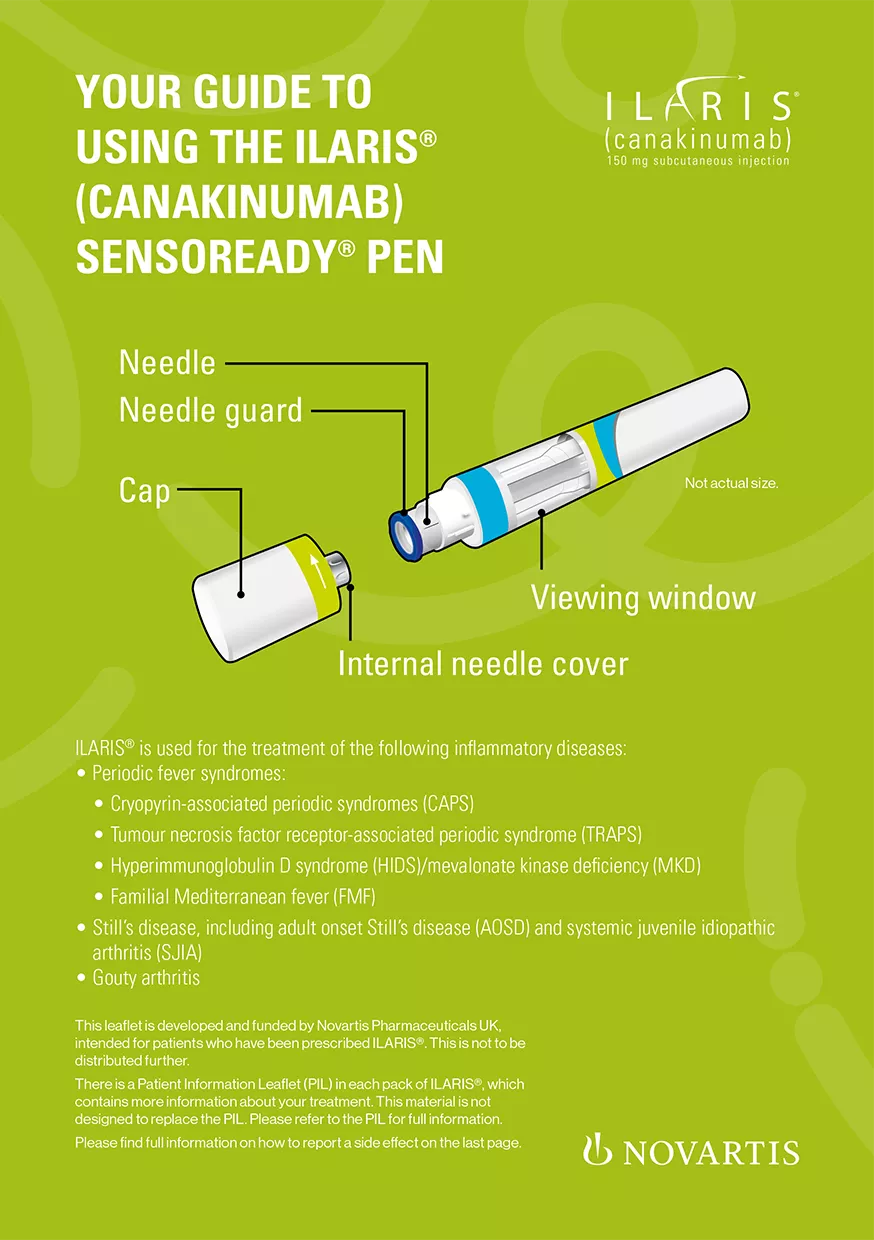
Patient guide to using the ILARIS Sensoready® pen
A booklet containing important information to know before starting treatment with the Ilaris Sensoready® pen, as well as step-by-step instructions for using the device correctly.
AOSD, adult-onset Still's disease; CAPS, cryopyrin-associated periodic syndromes; CINCA, chronic infantile neurological, cutaneous, articular syndrome; FCAS, familial cold autoinflammatory syndrome; FCU, familial cold urticaria; FMF, familial Mediterranean fever; HCP, healthcare professional; HIDS, hyperimmunoglobulin D syndrome; MKD, mevalonate kinase deficiency; MWS, Muckle-Wells syndrome; NOMID, neonatal-onset multisystem inflammatory disease; NSAID, non-steroidal anti-inflammatory drug; SJIA, systemic juvenile idiopathic arthritis; TRAPS, tumour necrosis factor receptor-associated periodic syndrome.
Reference
ILARIS® (canakinumab) Summary of Product Characteristics.
UK | September 2025 | FA-11307389-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.