Biblioteca
Lecturas recomendadas
Inclisiran y otros tratamientos hipolipemiantes
2022: the year in cardiovascular disease - the year of upfront lipid lowering combination therapy
Inclisiran and cardiovascular events: a patient-level analysis of phase III trials
Oligonucleotide therapeutics - a new class of cholesterol-lowering drugs
Khvorova A. N Engl J Med. 2017;376(1): 4-7.
Inclisiran - New hope in the management of lipid disorders?
Dyrbuś K, et al. J Clin Lipidol. 2020;14(1):16–27.
Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting
Soppert J, et al. Adv Drug Deliv Rev. 2020;S0169-409X(20)30103-4. doi:10.1016/j.addr.2020.07.019 [published online ahead of print, 2020 Jul 27].
Progress and prospects of biological approaches targeting PCSK9 for cholesterol-lowering, from molecular mechanism to clinical efficacy
Mohammad Malvandi A, et al. Expert Opinion on Biological Therapy. 2020. DOI: 10.1080/14712598.2020.1801628.
Targeting RNA With Antisense Oligonucleotides and Small Interfering RNA in Dyslipidemias
Katzmann JL, et al. J Am Coll Cardiol. 2020 Aug, 76 (5) 563-579.
Inclisiran for the Treatment of Cardiovascular Disease: A Short Review on the Emerging Data and Therapeutic Potential.
Kosmas CE, Muñoz Estrella A, Skavdis A, Peña Genao E, Martinez I, Guzman E. Ther Clin Risk Manag. 2020 Oct 28;16:1031-1037. doi: 10.2147/TCRM.S230592. PMID: 33149595; PMCID: PMC7604242.
Programa de desarrollo ORION
Lipid lowering with inclisiran: a real-world single-centre experience
Efficacy and Safety of Inclisiran in Patients with Polyvascular Disease: Pooled, Post Hoc Analysis of the ORION-9, ORION-10, and ORION-11 Phase 3 Randomized Controlled Trials | SpringerLink
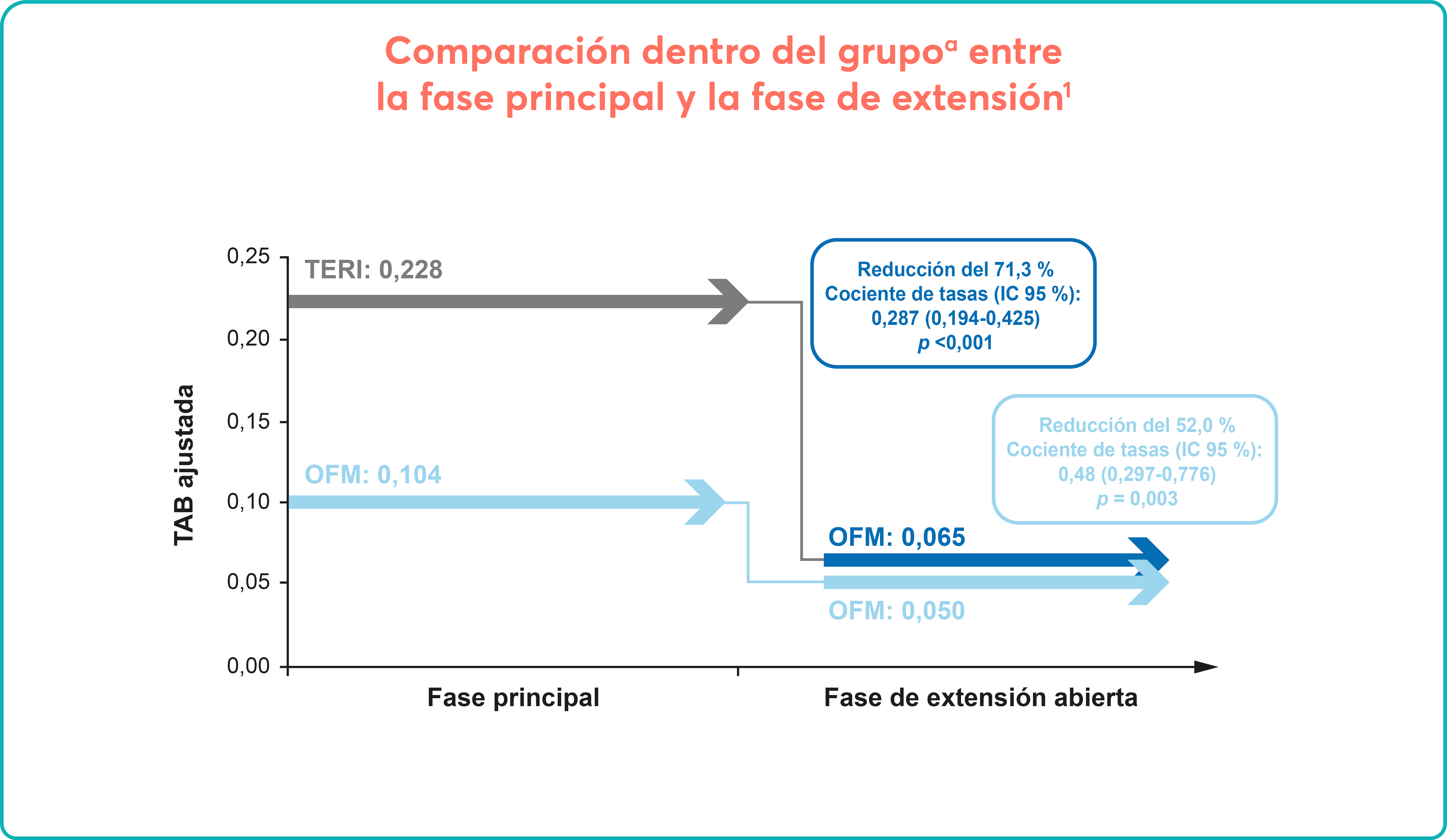
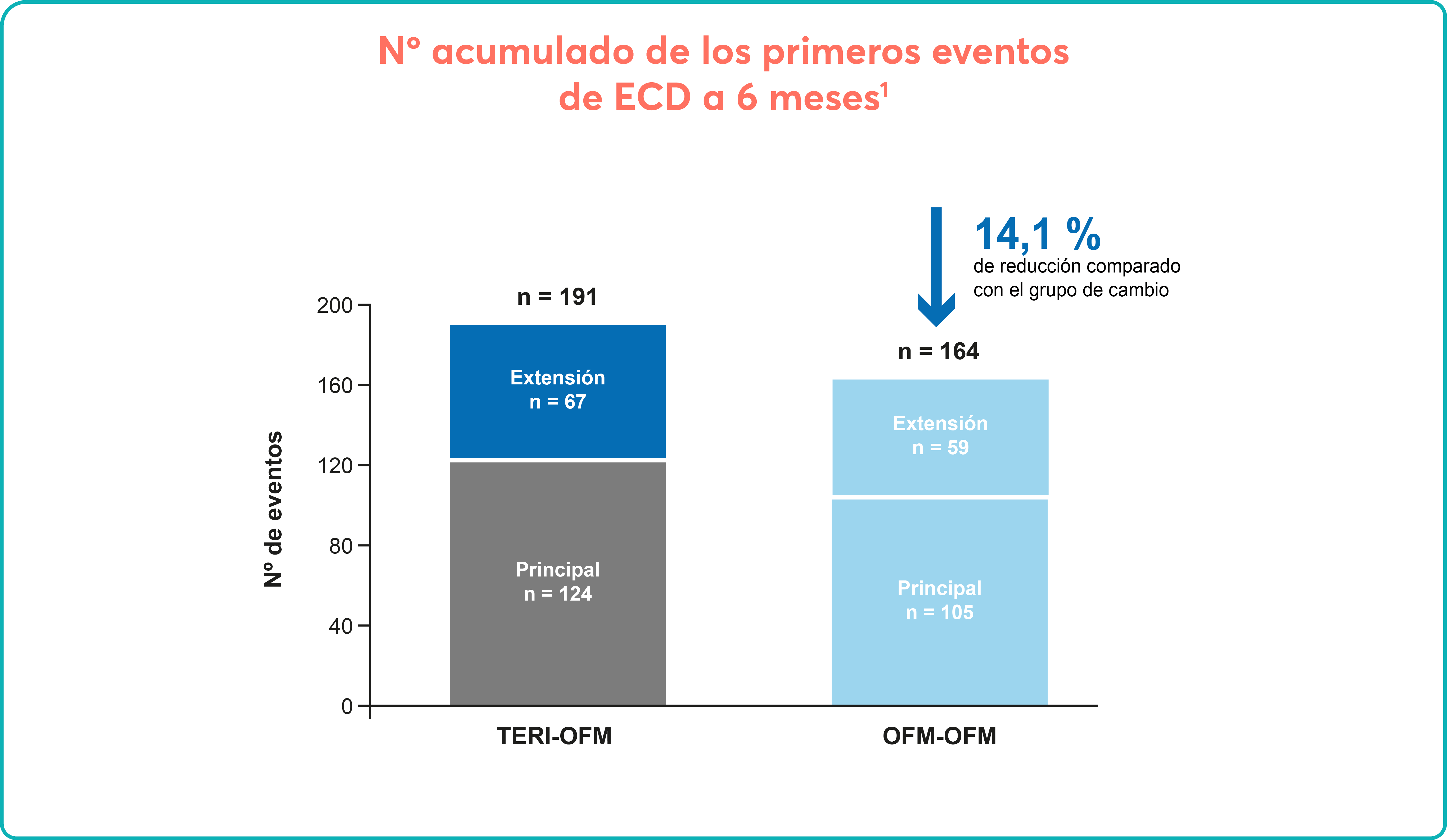
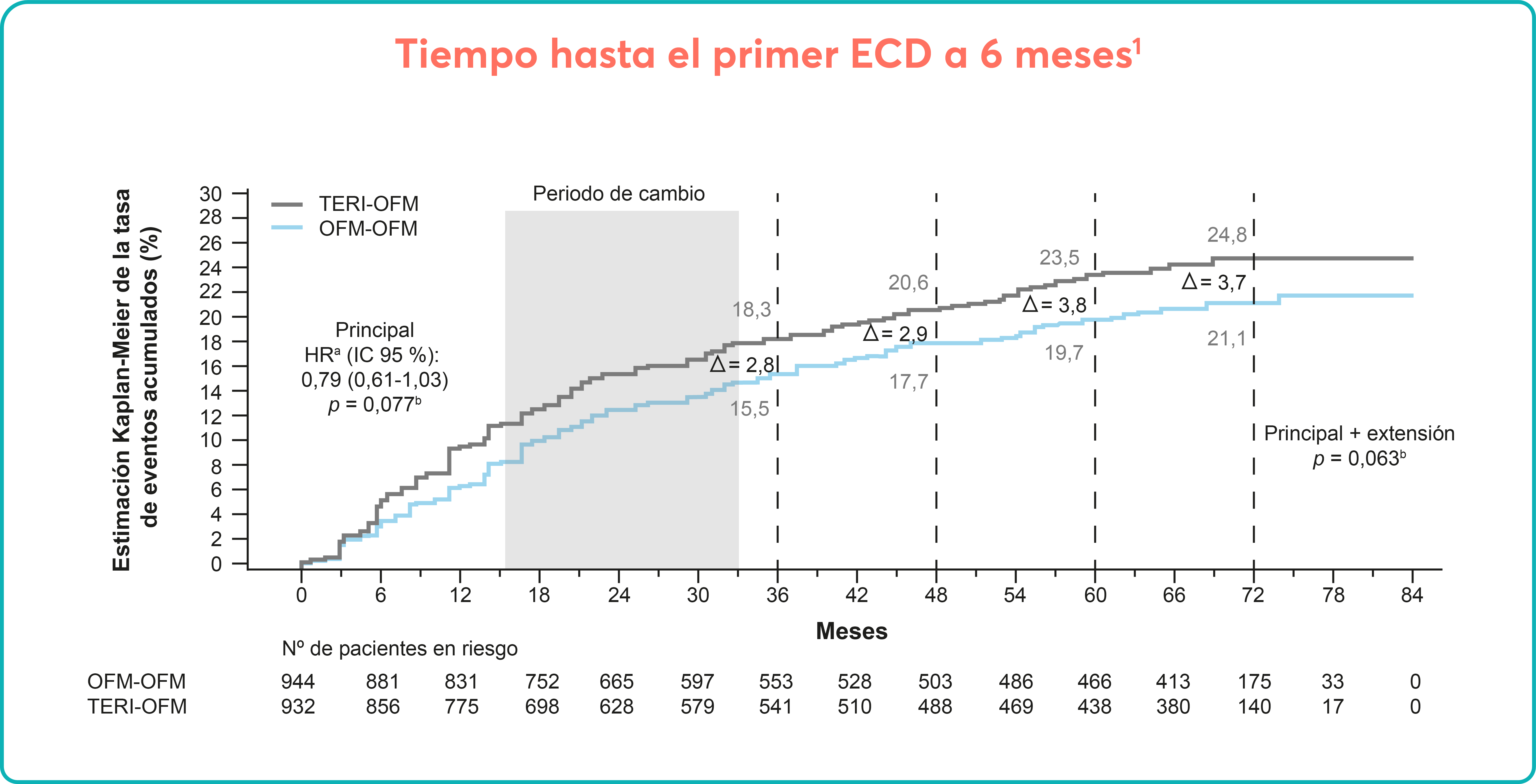
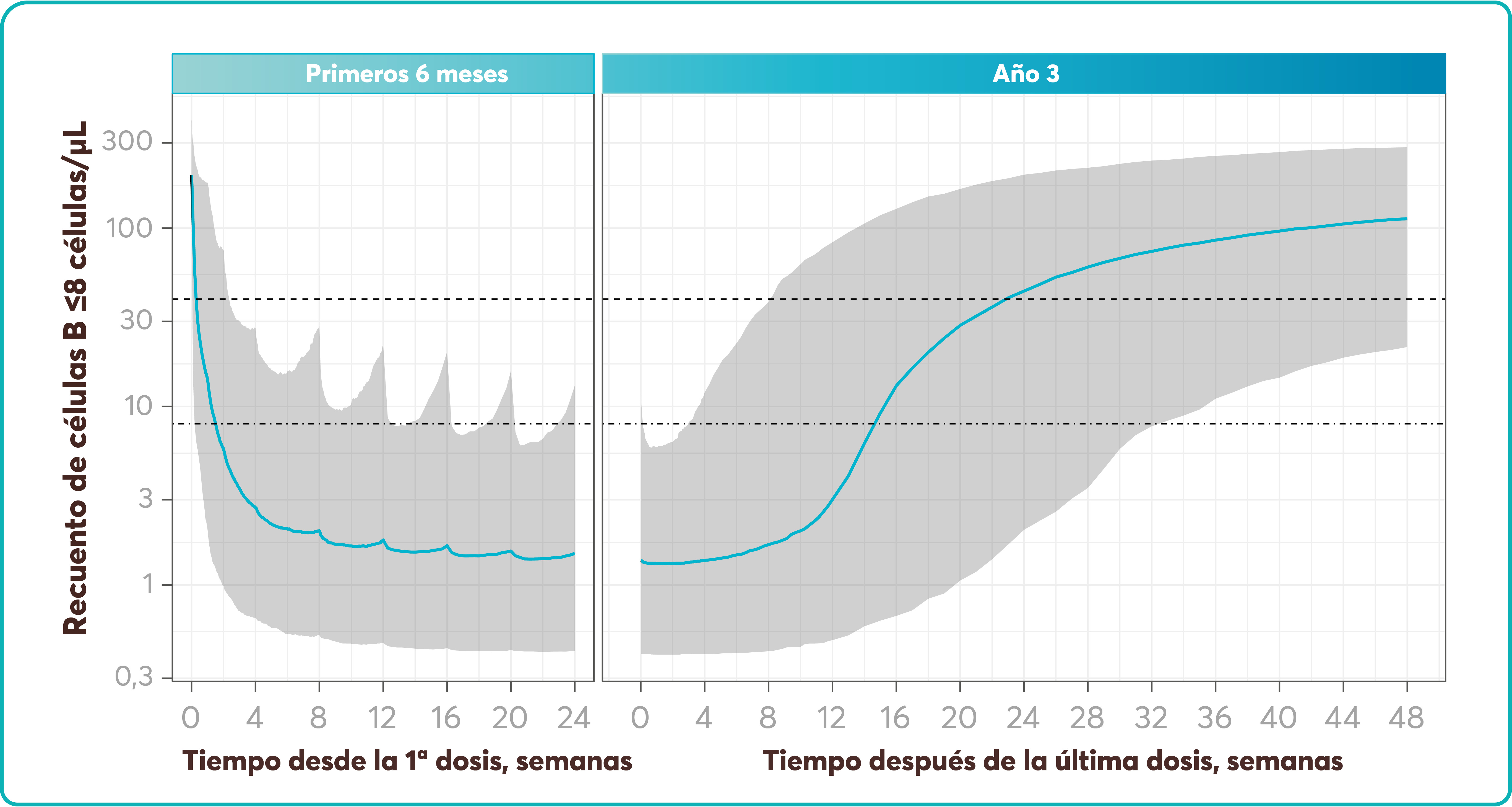
Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial - The Lancet Diabetes & Endocrinology
Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia
Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, Wijngaard PLJ, Curcio D, Jaros MJ, Leiter LA, Kastelein JJP; ORION-9 Investigators. N Engl J Med. 2020 Apr 16;382(16):1520-1530. doi: 10.1056/NEJMoa1913805. Epub 2020 Mar 18. PMID: 32197277.
Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol
Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, Kastelein JJP; ORION-10 and ORION-11 Investigators. N Engl J Med. 2020 Apr 16;382(16):1507-1519. doi: 10.1056/NEJMoa1912387. Epub 2020 Mar 18. PMID: 32187462.
Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol
Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, Hall T, Troquay RP, Turner T, Visseren FL, Wijngaard P, Wright RS, Kastelein JJ. N Engl J Med. 2017 Apr 13;376(15):1430-1440. doi: 10.1056/NEJMoa1615758. Epub 2017 Mar 17. PMID: 28306389.
Effects of Renal Impairment on the Pharmacokinetics, Efficacy, and Safety of Inclisiran: An Analysis of the ORION-7 and ORION-1 Studies
Wright RS, Collins MG, Stoekenbroek RM, Robson R, Wijngaard PLJ, Landmesser U, Leiter LA, Kastelein JJP, Ray KK, Kallend D. Mayo Clin Proc. 2020 Jan;95(1):77-89. doi: 10.1016/j.mayocp.2019.08.021. Epub 2019 Oct 17. PMID: 31630870.
Effect of 1 or 2 Doses of Inclisiran on Low-Density Lipoprotein Cholesterol Levels: One-Year Follow-up of the ORION-1 Randomized Clinical Trial.
Ray KK, Stoekenbroek RM, Kallend D, Nishikido T, Leiter LA, Landmesser U, Wright RS, Wijngaard PLJ, Kastelein JJP. JAMA Cardiol. 2019 Nov 1;4(11):1067-1075. doi: 10.1001/jamacardio.2019.3502. PMID: 31553410; PMCID: PMC6763983.
Highly Durable RNAi Therapeutic Inhibitor of PCSK9
Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, Wijngaard P, Horton JD, Taubel J, Brooks A, Fernando C, Kauffman RS, Kallend D, Vaishnaw A, Simon A. A. N Engl J Med. 2017 Jan 5;376(1):41-51. doi: 10.1056/NEJMoa1609243. Epub 2016 Nov 13. PMID: 27959715; PMCID: PMC5778873.
Clinical implications and outcomes of the ORION Phase III trials
Brandts J, Ray KK. Future Cardiol. 2020 Dec 21. doi: 10.2217/fca-2020-0150. Epub ahead of print. PMID: 33345605.
Terapias basadas en oligonucleótidos
The growth of siRNA-based therapeutics: Updated clinical studies
Zhang MM, Bahal R, Rasmussen TP, Manautou JE, Zhong XB. Biochem Pharmacol. 2021 Jan 26:114432. doi: 10.1016/j.bcp.2021.114432. Epub ahead of print. PMID: 33513339.
Advances in oligonucleotide drug delivery
Roberts TC, Langer R, Wood MJA. Nat Rev Drug Discov [Internet]. 2020.
Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug
Debacker AJ, et al. Mol Ther. 2020 Aug 5;28(8):1759-1771. doi: 10.1016/j.ymthe.2020.06.015. Epub 2020 Jun 17. PMID: 32592692; PMCID: PMC7403466.
Antisense oligonucleotides and other genetic therapies made simple
Rossor AM, Reilly MM, Sleigh JN. Pract Neurol. 2018;18(2):126–31.

MSL Online
Pide tu cita con el departamento médico.