
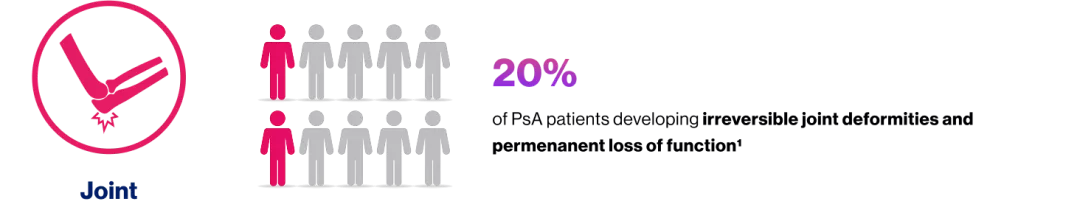
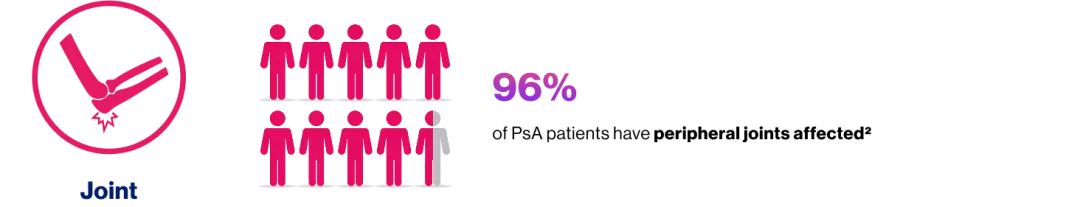
Active PsA
IR to csDMARD(s) and biologic-naïve
US detected synovitis
≥1 clinical enthesitis (SPARCC enthesitis index)
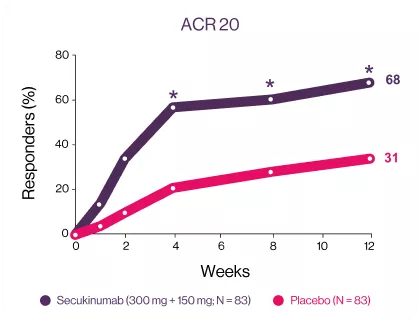
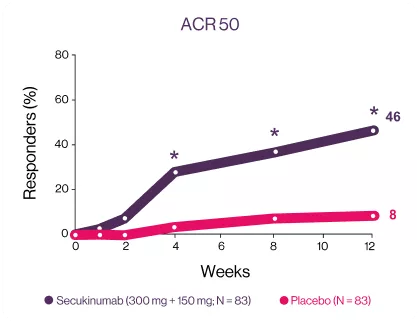
Cosentyx significantly has higher ACR response rate at week 12 starting early from week 43
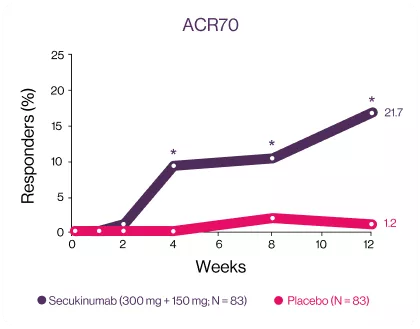
ACR20 and ACR50 responses were met and favored secukinumab-treated patients against placebo at week 12, with significant improvements observed as early as week 1 for ACR20 and week 2 for ACR50 compared with placebo3 | Significantly higher responses were observed in secukinumab-treated patients for the exploratory endpoints (ACR70 response) at week 12 compared with placebo.3 | |
Image
| Image
| Image
|
*P<0.05 versus placebo. (A) ACR20 response (NRI, odds ratio [95% CI]: 5 [2; 9], P<0.0001, relative risk: 2); (B) ACR50 response (NRI, odds ratio [95% CI]: 10 [4; 24], P<0.0001, relative risk. | *P<0.05 versus placebo. (A) ACR20 response (NRI, odds ratio [95% CI]: 5 [2; 9], P<0.0001, relative risk: 2); (B) ACR50 response (NRI, odds ratio [95% CI]: 10 [4; 24], P<0.0001, relative risk. | *ACR70 response (NRI, odds ratio [95% CI]: 23 [3; 178], P=0.0013, relative risk: 18).NRI, non responders’ imputation. |
ULTIMATE: Study Design.3
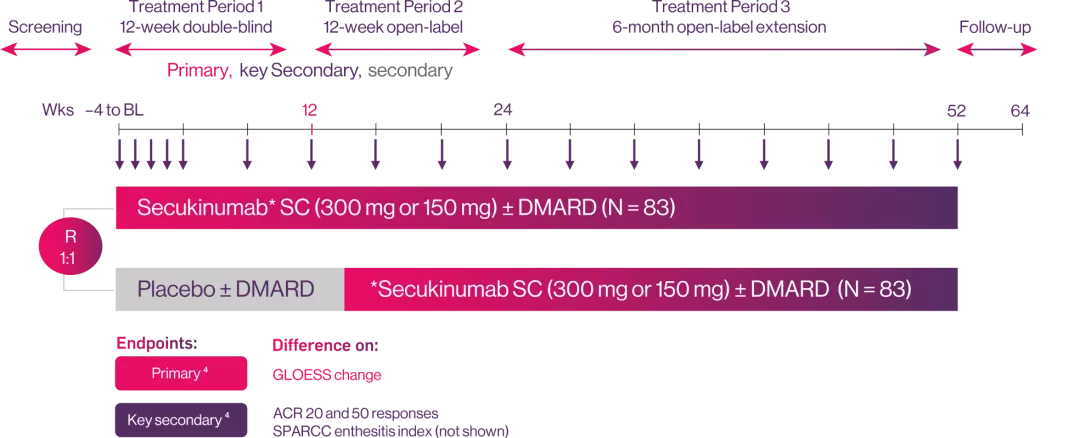
Study population
Randomisation:
weekly secukinumab (150 or 300 mg by severity of psoriasis) or placebo followed by 4-weekly dosing thereafter
Objective:
To investigate the dynamics of response of synovitis to IL-17A inhibition with secukinumab in patients with active PsA using Power Doppler ultrasound.
Cosentyx® provides maintained improvements in signs and symptoms of PsA5
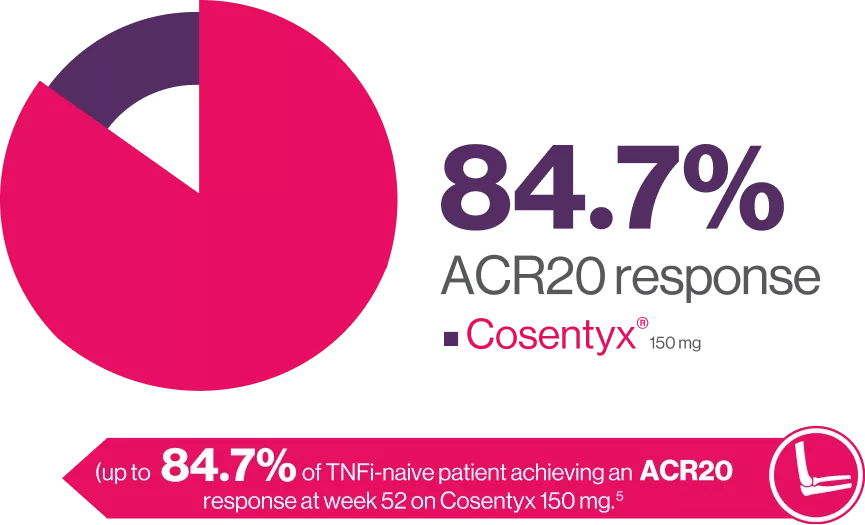
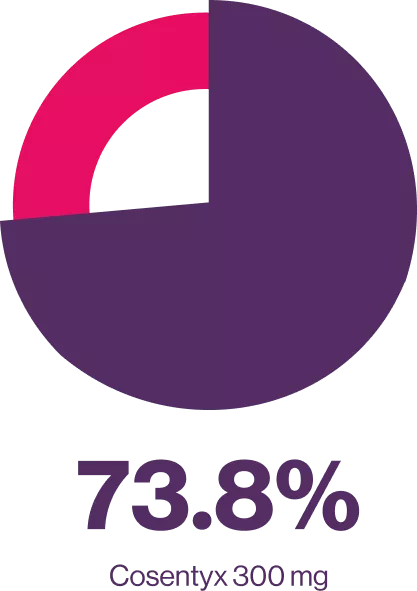
FUTURE 2 is a 5-year, randomized, double-blind, multicenter, placebo-controlled, parallel-group study. Briefly, patients were ≥ 18 years old with active PsA. Eligible patients were randomized (1:1:1:1) to receive subcutaneous doses of secukinumab 300 mg, 150 mg, 75 mg, or placebo at baseline, weeks 1, 2, 3, and 4, and every 4 weeks thereafter. At Week 16, placebo-treated patients were re-randomized (1:1) to receive secukinumab 300 mg or 150 mg sc from Week 16 (if they had < 20 % improvement from baseline in tender and swollen joint counts) or Week 24. Here we describe efficacy outcomes in patients naive to TNFi therapy (TNFi-naive) and those previously treated with TNFi (TNFi-exposed).5
Cosentyx® provides improvements in symptoms of PsA6
ACR20 Response at WEEK 104
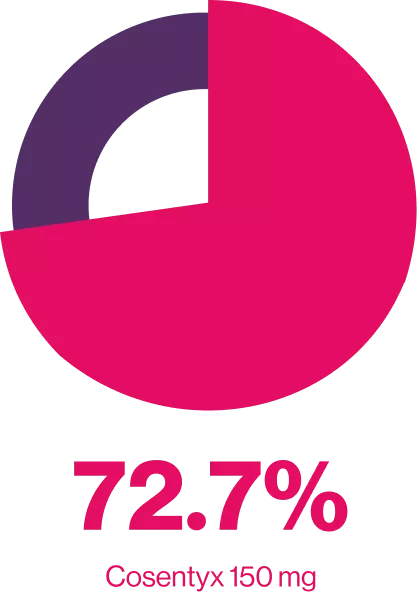
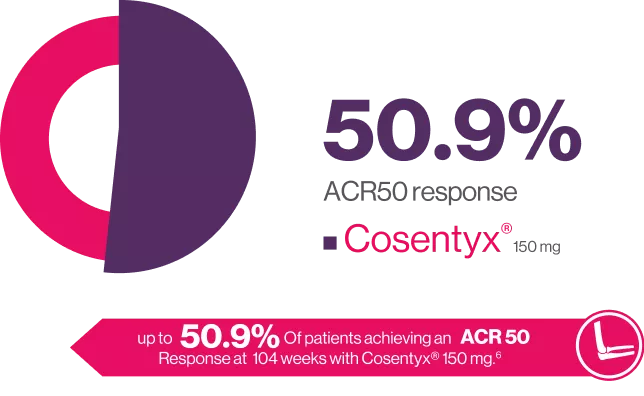
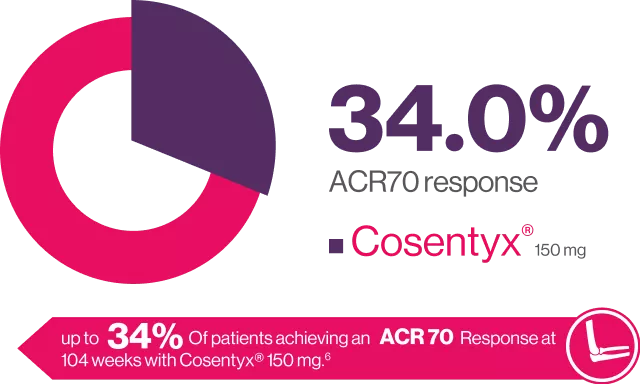
FUTURE 2 is an ongoing. multicenter, randomized, double-blind, parallel group, Placebo-controlled study the design of which has been published in detail in brief 397 patients with active PsA were randomized (1:1:1:1) to receive Cosentyx” (300, 150 or 75 mg) or placebo at baseline, weeks 1, 2, 3 and 4 and every 4 weeks thereafter. to assess long-term efficacy, safety of secukinumab up to 104 weeks in patients with active PsA.6

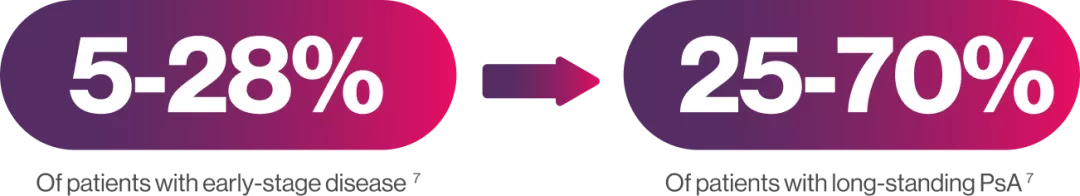
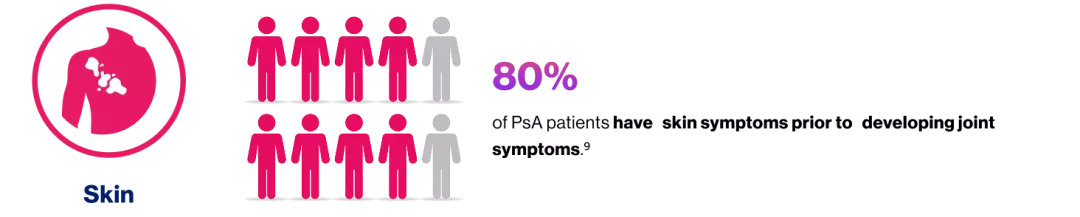
The prevalence of axial disease in patients with PsA varies with disease duration and the definition used, occurring in7:
Cosentyx 300 mg and 150 mg significantly improved ASAS20 vs placebo at week 12.7
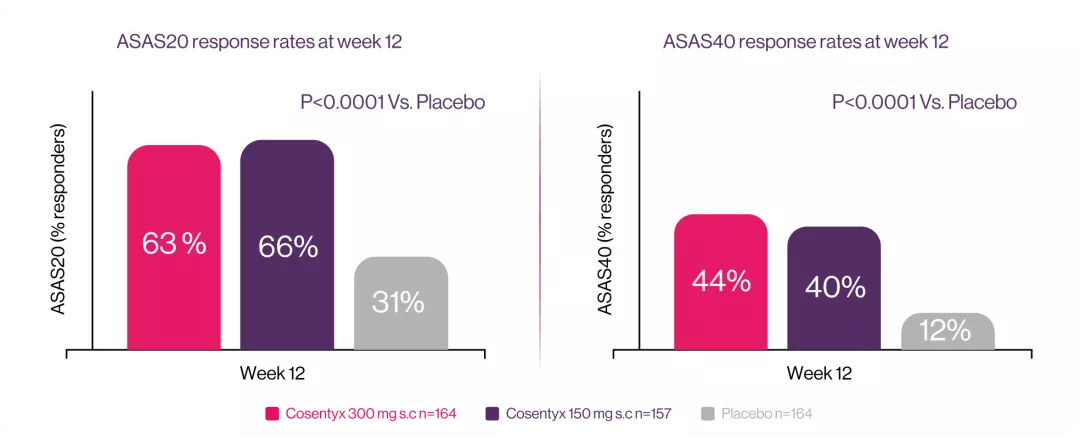
MAXIMISE: was a phase 3b, double-blind, placebo-controlled, multi-centre 52-week trial that include 498 patients. The trial consisted of two treatment periods; a placebo-controlled period from baseline to week 12 followed by an active treatment period from week 12 to 52. Eligible patients were randomised (1:1:1) to subcutaneous (s.c.) secukinumab300 mg, 150 mg or placebo weekly for 4 weeks and every 4 weeks thereafter. The objective of the MAXIMISE trial was to specifically evaluate the efficacy and safety of secukinumab 300 mg and 150 mg in managing axial manifestations in patients with PsA with an inadequate response to non-steroidal anti inflammatory drugs (NSAIDs).7
Cosentyx® offers your patients structural benefits
Patients with no radiographic structural progression at week 24
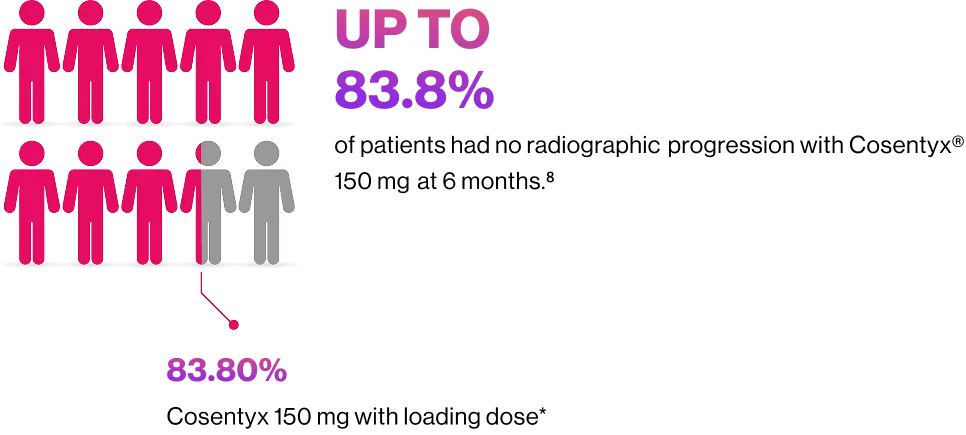
*vs.Cosentyx® 150 mg without loading dose in the placebo group (73.6%).
Cosentyx® led to significant improvements in skin clearance at week 16.10
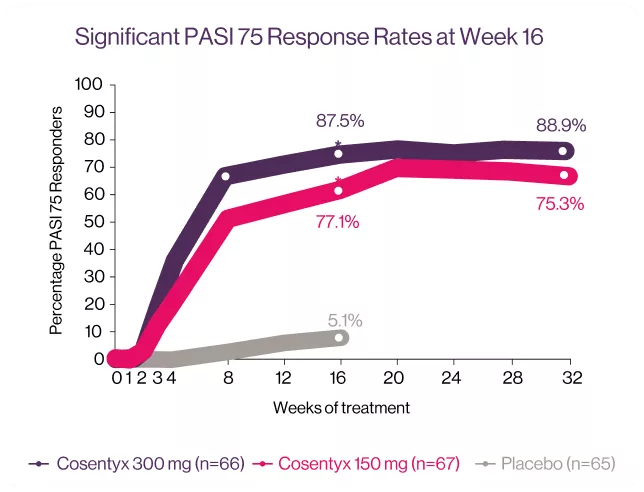
*P < 0001 vs. placebo (P-values available for week 16 only).10
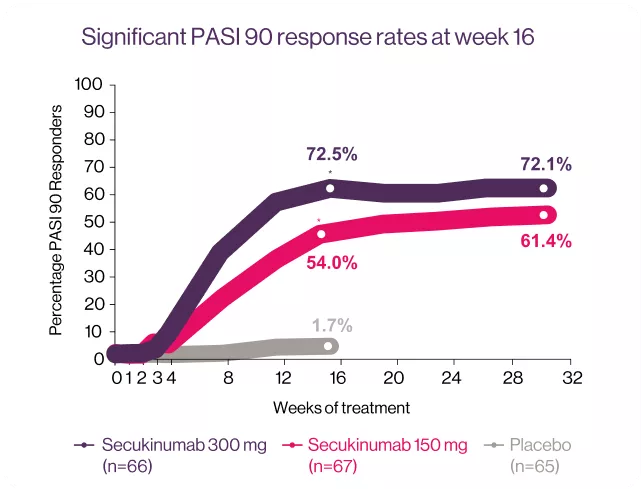
*P < 0001 vs. placebo (P-values available for week 16 only).10
TRANSFIGURE: Is a randomized, double-blind, placebo-controlled trial, conducted across 39 study sites in Australia, Belgium, Czech Republic, Denmark, Germany, Greece, Spain, U.K. and U.S.A. The study consisted of a screening period of up to 6 weeks; a 16-week treatment period until the primary end point, followed by continued treatment to 132 weeks; and an 8-week treatment free follow-up period. In total 198 patients were randomized to the three study groups: Cosentyx® 300 mg (n=66), Cosentyx® 150 mg (n=67) and placebo (n=65). The primary objective was to demonstrate the superiority of Cosentyx® over placebo, as assessed by the percentage change in total fingrenail NAPSI score from baseline to week 16.10

Cosentyx® provided improvements across multiple domains of PsA including signs and symptoms in patients with active disease through 3 years of treatment.

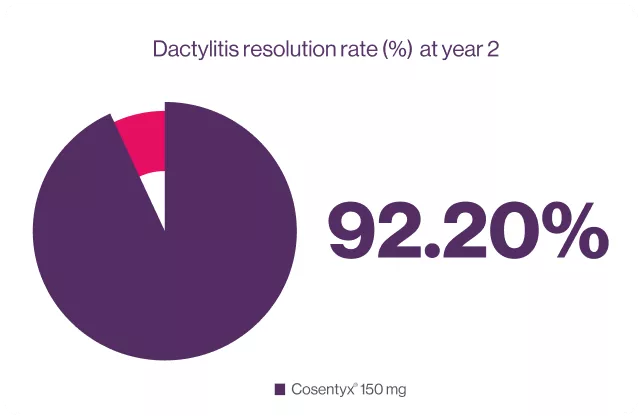
*Resolution of enthesitis are shown for patients with these symptoms at baseline (enthesitis: N=99 (150 mg)). Results are mean±SD unless otherwise stated.11

92.20% Dactylitis resolution rate was observed in patients treated with cosentyx® 150 mg.6
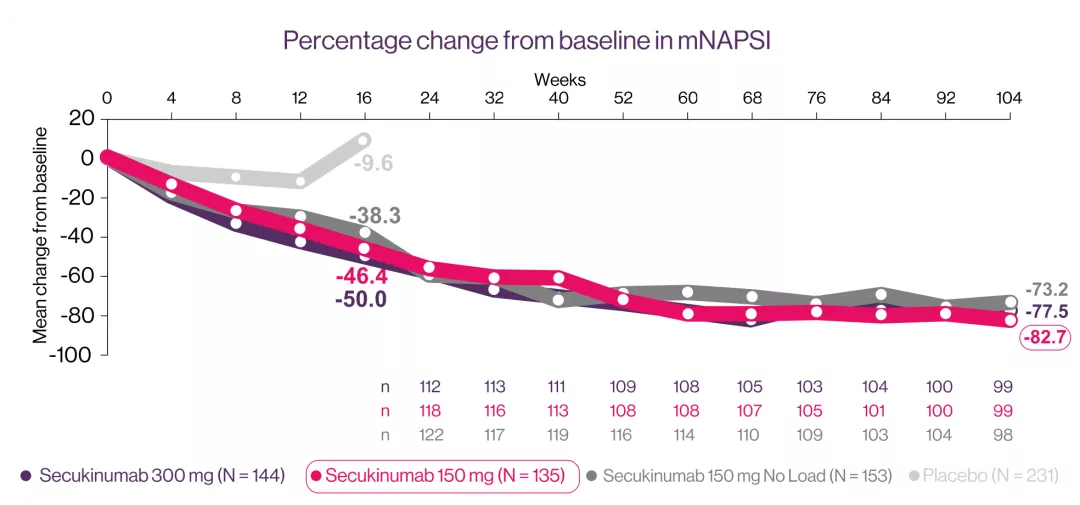
Cosentyx® reduced NAPSI score at week 16 and up to week 10412*
Non-Significant Difference
*At Week 104, the mean percentage mNAPSI changes were -77.5%, -82.7% and -73.2% for patients originally randomized to 300 mg, 150 mg and 150 mg no load, respectively.
FUTURE 5 was a 2-year, randomised, double-blind, placebo-controlled, parallel-group Phase III study. Patients (n=996) aged ≥18 years and who met the CASPAR criteria with active PsA at screening were randomly (2:2:2:3) allocated to one of four treatment groups: secukinumab 300 mg loading dose at Weeks 0, 1, 2 and 3 followed by dosing every 4 weeks (q4w) starting at Week 4; secukinumab 150 mg loading dose at Weeks 0, 1, 2 and 3 followed by dosing q4w starting at Week 4; secukinumab 150 mg no-load received secukinumab 150 mg treatment at baseline and placebo at Weeks 1, 2 and 3 followed by secukinumab 150 mg q4w from Week 4; or matched placebo, all self-administered subcutaneous (s.c.)
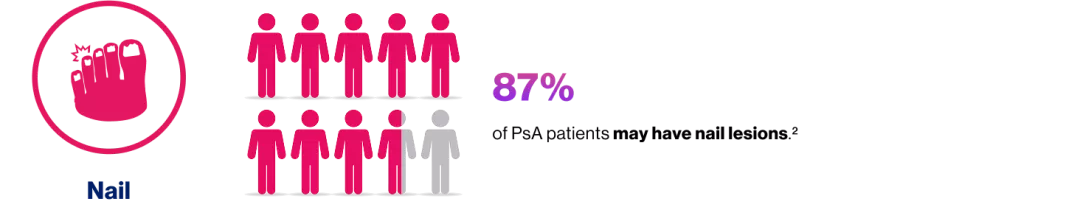
Efficacy assessments through Week 104 in the nail subset included mean change from baseline in fingernail mNAPSI scores, percentage change from baseline in fingernail mNAPSI, proportion of patients achieving mNAPSI score of ≤2 (in patients with baseline mNAPSI score of >2), proportion of patients achieving ≥20/50% improvement according to American College of Rheumatology response composite index (ACR20/ACR 50) and ≥ 90% improvement in Psoriasis Area and Severity Index (PASI 90) responses and proportion of patients with resolution of dactylitis (as defined by the Leeds Dactylitis Index) and enthesitis (as defined by the Leeds Enthesitis Index).12
BL: baseline, csDMARD: Conventional synthetic disease-modifying antirheumatic drugs, ACR20: American Collage of Rheumatology response criteria, PsA: psoriatic arthritis , MAXIMISE: Managing AXIal manifestations in psoriatic arthritis with Secukinumab, bDMARD: Biological Disease Modifying Anti-Rheumatic Drugs, ASAS: Assessment of Spondyloarthritis international Society, S.C: Subcutaneous, GRAPPA: Group for Research and Assessment of Psoriasis and PsA, IBD: inflammatory bowel disease, MRI: magnetic resonance imaging, PASI: Psoriasis Area and Severity Index , NAPSI: Nail Psoriasis Severity Index, mNAPSI: Modified Nail Psoriasis Severity Index .
Cosentyx API
Cosentyx API
References
Tucker LJ, Ye W, Coates LC. Novel concepts in psoriatic arthritis management: can we treat to target?. Current rheumatology reports. 2018 Nov;20(11):1-9.
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. New England Journal of Medicine. 2017 Mar 9;376(10):957-70.
D’Agostino MA, Schett G, López-Rdz A, et al. Response to secukinumab on synovitis using Power Doppler ultrasound in psoriatic arthritis: 12-week results from a phase III study, ULTIMATE. Rheumatology. 2022 May;61(5):1867-76.
Supplemenatry appendix of: D’Agostino MA, Schett G, López-Rdz A, et al. Response to secukinumab on synovitis using Power Doppler ultrasound in psoriatic arthritis: 12-week results from a phase III study, ULTIMATE. Rheumatology. 2022 May;61(5):1867-76.
Kavanaugh A, McInnes IB, Mease PJ, Hall S, Chinoy H, Kivitz AJ, Wang Z, Mpofu S. Efficacy of subcutaneous secukinumab in patients with active psoriatic arthritis stratified by prior tumor necrosis factor inhibitor use: results from the randomized placebo-controlled FUTURE 2 study. The Journal of rheumatology. 2016 Sep 1;43(9):1713-7.
McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B, Kajekar R, Delicha EM, Pricop L, Mpofu S. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2-year results from the phase 3 FUTURE 2 study. Rheumatology. 2017 Nov 1;56(11):1993-2003.
Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Annals of the rheumatic diseases. 2021 May 1;80(5):582-90.
Mease P, van der Heijde D, Landewé R, Mpofu S, Rahman P, Tahir H, Singhal A, Boettcher E, Navarra S, Meiser K, Readie A. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Annals of the rheumatic diseases. 2018 Jun 1;77(6):890-7.
Gottlieb AB, Mease PJ, Mark Jackson J, et al. Clinical characteristics of psoriatic arthritis and psoriasis in dermatologists' oces. Journal of dermatological treatment. 2006 Jan 1;17(5):279-87.
Reich K, Sullivan J, Arenberger P, Mrowietz U, Jazayeri S, Augustin M, Parneix A, Regnault P, You R, Milutinovic M. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. British Journal of Dermatology. 2019 Nov;181(5):954-66.
Mease PJ, Kavanaugh A, Reimold A, et al. Secukinumab in the treatment of psoriatic arthritis: efficacy and safety results through 3 years from the year 1 extension of the randomised phase III FUTURE 1 trial. RMD open. 2018 Aug 1;4(2):e000723.
Nash P, Mease PJ, Kirkham B, Singhal A, Quebe-Fehling E, Pricop L, Gaillez C. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clinical and experimental rheumatology. 2021 Sep 3.
Approved by Egyptian Drug Authority:BF0424OA4792/092025. Invalidation date: 06/12/2026.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
BF0424OA4792/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
