
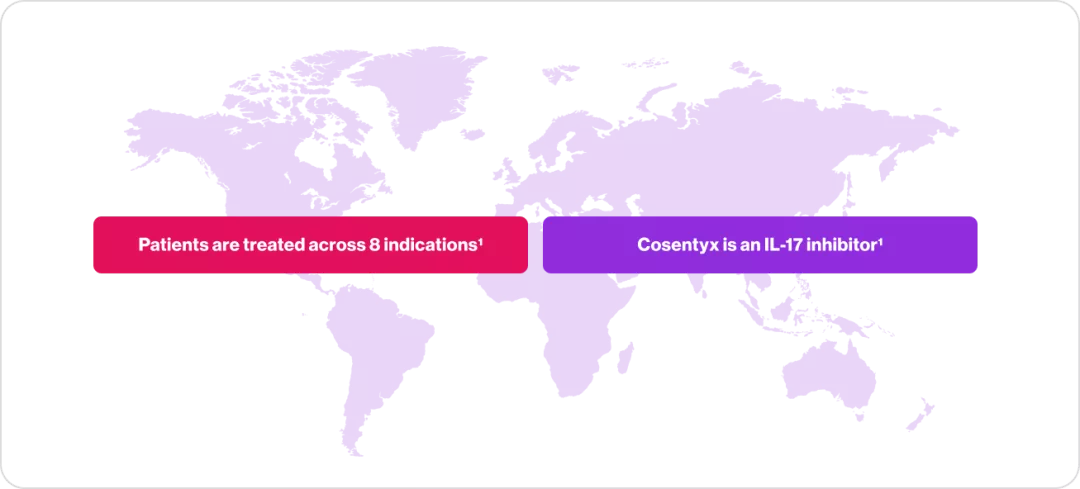
Cosentyx is indicated for Adult Plaque psoriasis (PsO) ,Pediatric PsO ,Hidradenitis suppurativa (HS), Psoriatic arthritis (PsA) ,Axial spondyloarthritis (axSpA), Ankylosing spondylitis (AS), Non-radiographic axial spondyloarthritis (nr-axSpA), Juvenile idiopathic arthritis (JIA), Enthesitis-related arthritis (ERA), Juvenile PsA (JPsA)1
Effective relief at all 6 GRAPPA manifestations with Cosentyx (secukinumab)®2
IL-17A plays a key role:
In the pathogenesis of plaque psoriasis, psoriatic arthritis and axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis)1

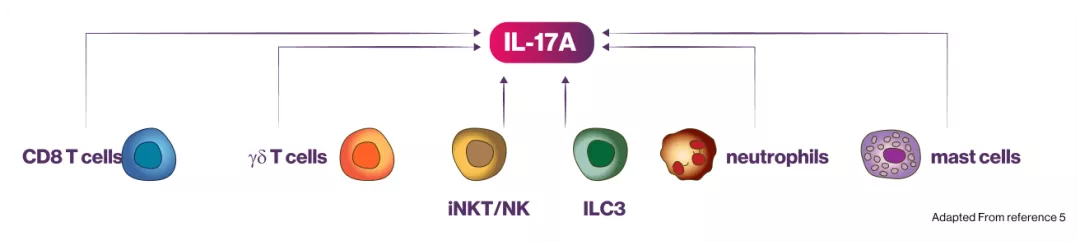
Cosentyx blocks IL-17A, a key cytokine responsible for inflammation that underlies axSpA, PsA, PsO, and HS1
Increased levels of IL-17A are found in the affected tissues of patients with axSpA, PsA, PsO, HS1
Image

| Across 8 indications1 |








All in one relief from the multiple manifestation of PsA
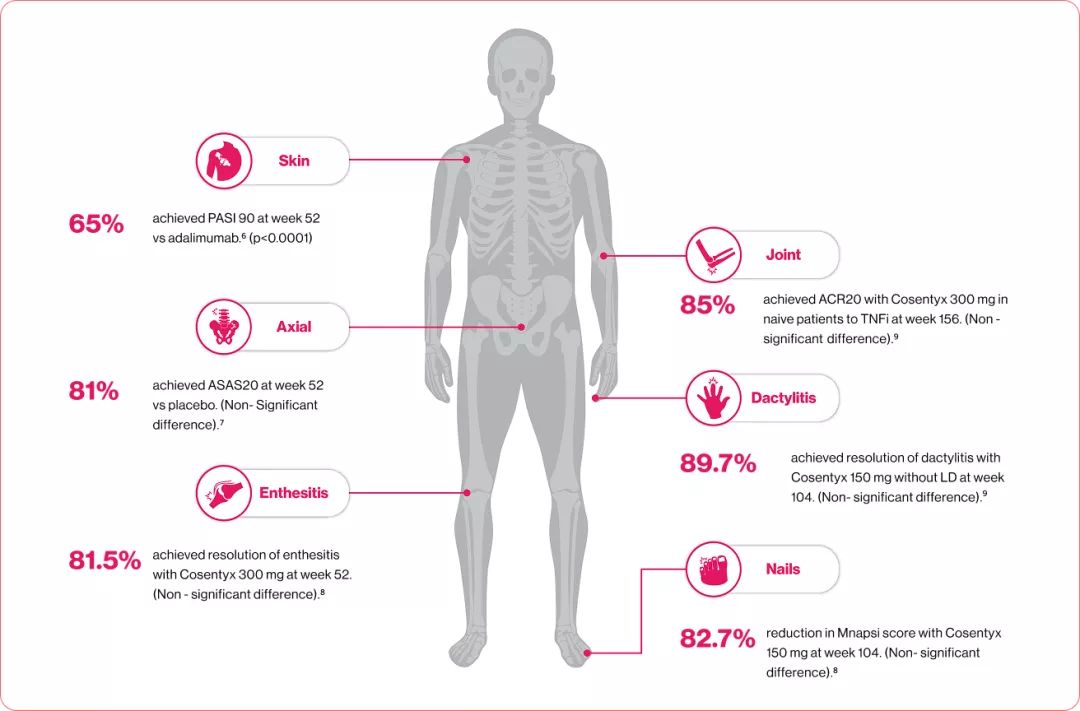
IL: Interleukin, PsA: Psoriatic arthritis, MOD: Mode of disease, MOA: Mode of Action, CD: Cluster of differentiation, iNKT: invariant natural killer T, NK: Natural Killer cell, ILC: Innate Lymphoid cells, PsA: Psoriatic arthritis, TNF: Tumor necrosis factor, PASI: Psoriatic arithritis severity index, ASAS: Assessment of SpondyloArthritis International Society, ACR: American College of Rheumatology, TNFi:Tumor necrosis factor inhibitor, LD: Loading dose, Mnapsi: Modified Nail Psoriasis Severity Index.
Cosentyx API
Cosentyx API
References
Egyptian Drug Authority (EDA), Cosentyx leaflet approval date: 23/03/2025.
Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nature Reviews Rheumatology. 2022 Aug;18(8):465-79.
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. New England Journal of Medicine. 2017;376(10):957-70.
Gottlieb AB, Mease PJ, Mark Jackson J,et al. Clinical characteristics of psoriatic arthritis and psoriasis in dermatologists' offices. Journal of dermatological treatment. 2006;17(5):279-87.
Brembilla NC, Senra L, Boehncke WH. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Frontiers in immunology. 2018;9:1682.
McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. The Lancet. 2020 May 9;395(10235):1496-505.
Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Annals of the rheumatic diseases. 2021 May 1;80(5):582-90.
Nash P, Mease PJ, Kirkham B, et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clinical and experimental rheumatology. 2021 Sep 3.
McInnes IB, Mease PJ, Kivitz AJ, et al. Long-term efficacy and safety of secukinumab in patients with psoriatic arthritis: 5. year (end-of-study) results from the phase 3 FUTURE 2 study. The Lancet Rheumatology. 2020 Apr 1;2(4):e227-35.
Approved by Egyptian Drug Authority:BF0424OA4792/092025. Invalidation date: 06/12/2026.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
BF0424OA4792/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |



