
Cosentyx demonstrated a safety profile over long-term treatment in patients with PsA.1
Data on a year-by-year basis for any AE, any SAE, serious infections, Candida infection, IBD, and MACE showed no increase with secukinumab treatment over time across studies within PSA.1
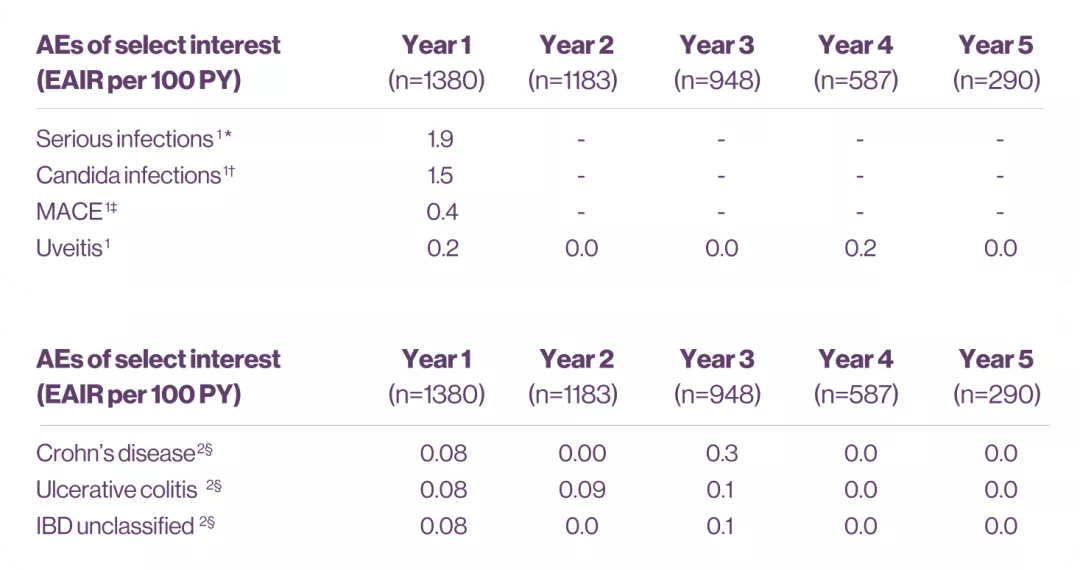
The cumulative reporting rate
of IBD remained stable at
approximately 0.20 reported
events per 100 PY.2
This long-term (up to 5 years) safety assessment provides a broader understanding of the safety of
secukinumab and supports its long-term use in these chronic systemic inflammatory conditions.1
*Values are based on system organ class: infections and infestations.1
†Values are based on the high-level term.1
‡Values are based on Novartis MedDRA query, which comprises (1) any MI, (2) any CVA, and (3) all other CV events that are fatal, out of a listing of 2200+ terms.2
§Data are displayed to 2 decimals where N>1000; if N<1000, then data are displayed to 1 decimal.2
Each and every month, Your patients receive the treatment you prescribed
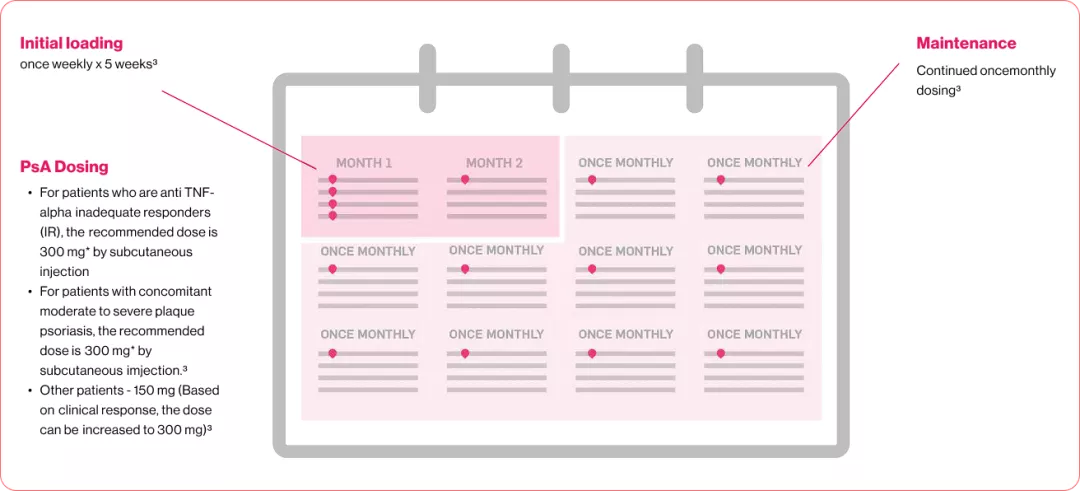
Adapted From reference 3
AE: Adverse event, CV: Cardiovascular, EAIR: Exposure-adjusted incidence rate, IBD: Inflammatory bowel disease, MACE: Major adverse cardiovascular event, MedDRA=Medical Dictionary for Regulatory Activities, MI: Myocardial infarction, PsA: Psoriatic arthritis, PsO: Plaque psoriasis, PY: Patient-years.
Cosentyx API
Cosentyx API
References
Deodhar A, Mease PJ, McInnes IB, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis research & therapy. 2019 Dec;21(1):1-1.
Schreiber S, Colombel JF, Feagan BG, et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Annals of the rheumatic diseases. 2019 Apr 1;78(4):473-9.
Egyptian Drug Authority (EDA), Cosentyx leaflet approval date: 23/03/2025.
Approved by Egyptian Drug Authority:BF0424OA4792/092025. Invalidation date: 06/12/2026.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
BF0424OA4792/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
