
Safety and adverse event management
“Specific adverse events are predictable and manageable with dose modification, here we will explore the most common Adverse events , rates of discontinuation or dose reduction for each, how to monitor these AEs and how to appropriately adjust the dose“
Dose reductions due to AEs

Discontinuation due to AEs

Most common AEs leading to discontinuation in the KISQALI arms
5 most common AEs in the KISQALI arms

Most common grade 3 or 4 AEs ( 5%) in the KISQALI arms

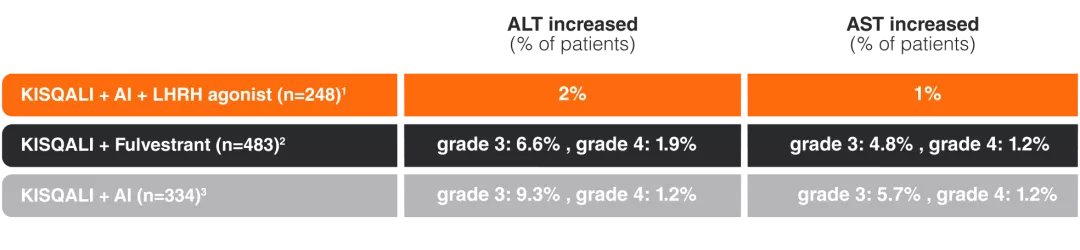
“KISQALI – Specific AEs were Predictable, Manageable with dose modification across all 3 trials4“
Neutropenia4
Image

| 75.4% all grades
|
Hepatobiliary toxicity4
Image

| 13.2% grades 3/4
|
QT interval prolongation4
Image

| 1.4% >500 msec
|
KISQALI—Optimize outcomes with early monitoring4
MULTIPLE GUIDELINES RECOMMEND MONITORING AS PART OF ROUTINE FOLLOW-UP CARE FOR MBC
- The European Society for Medical Oncology (ESMO 2024) recommends regular systematic monitoring as it facilitate communication between patients and their treatment teams about the toxicities of anticancer therapies. Reporting does not have to be tied to regular follow-up visits so that it may permit earlier introduction of ameliorative interventions and supportive care services.5
- Due to Cancer therapy-related cardiovascular toxicity the European Society of Cardiology (ESC) recommends ECG testing for all patients with advanced breast cancer receiving KISQALI before beginning treatment.6
RECOMMENDED MONITORING SCHEDULE4
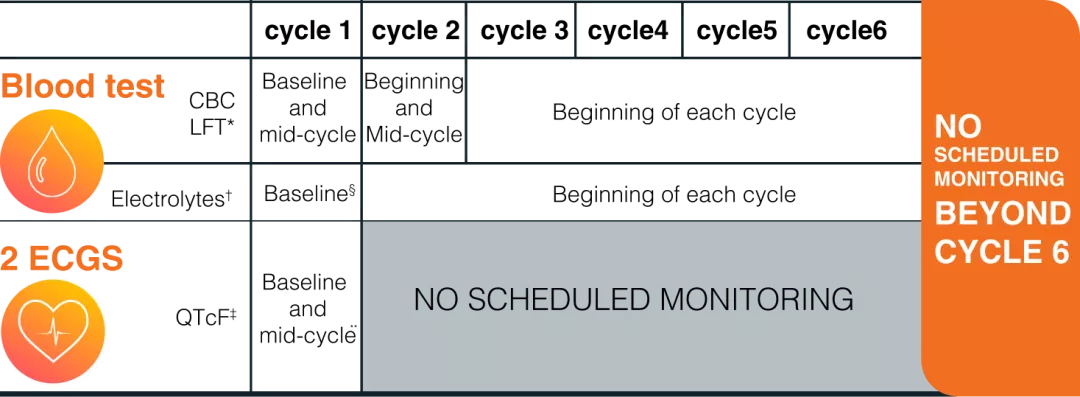
Any additional monitoring should be performed as clinically indicated4
* If grade 2 abnormalities are noted, more frequent monitoring is recommended.4 †Correct any electrolyte abnormalities prior to treatment4
‡ KISQALI should be only initiated in patients with QTcF <450 ms. In case of QTcF prolongation during therapy, more frequent ECG monitoring is recommended.4
**ECG should be repeated at approximately day 14 of the first cycle, then as clinically indicated4
§ Perform baseline assessment prior to treatment initiation.4
CBC, complete blood count; ECG, electrocardiogram; LFT, liver function test. QTcF, QT interval corrected by Fridericia›s fromula.Basic
KISQALI—Clear, straightforward dose adjustments4
DOSE MODIFICATIONS FOR SPECIFIC AEs4
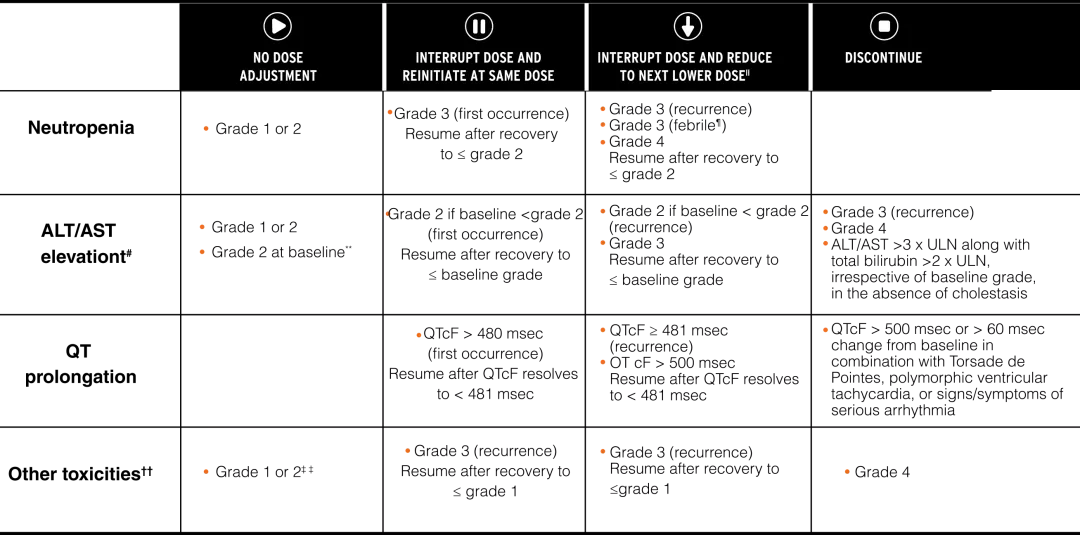
ll If dose reduction below 200 mg is required, discontinue treatment.4
¶ Grade 3 neutropenia with single episode of fever >38.3°C (or above 38°C for more than 1 hour and/or concurrent infection).4
# Without total bilirubin increase >2 x ULN.4
** Baseline=prior to initiation of treatment.4
†† Excluding neutropenia, hepatotoxicity, and QT interval prolongation.4
‡ ‡ Initiate appropriate medical therapy and monitor as clinically indicated.4
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events;
LLN, lower limit of normal; QTcF, QT interval corrected by Fridericia’s formula; TB, total bilirubin; ULN, upper limit of normal, AEs: Adverse events
AE, Adverse Event; AI, Aromatase Inhibitor; LHRH, luteinizing hormone-releasing hormone; ALT, alanine transaminase; AST, aspartate
aminotransferase; LFTs, Liver Function Tests; ULN, upper limit normal; QTcF,,QT using Fridericia's (cube root) correction; CBC, complete blood count; ECG, electrocardiogram
References
1.Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, Hurvitz SA, Chow L, Sohn J, Lee KS, Campos-Gomez S. Ribo ciclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MON ALEESA-7): a randomised phase 3 trial. The Lancet Oncology. 2018 Jul 1;19(7):904-15.
2.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín M, Nusch A. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. Journal of Clinical Oncology. 2018 Aug
20;36(24):2465-72.
3.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, André F, Winer EP, Janni W. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. New England journal of medicine. 2016 Nov 3;375(18):1738-48.
4-KISQALI. Summary of Products Characteristics (SMPC). available at: https://www.ema.europa.eu/en/documents/product-information/kisqali-epar-product-information_en.pdf last accessed 16/6/2025.
5-Cardoso F, Paluch-Shimon S, Schumacher-Wulf E, Matos L, Gelmon K, Aapro MS, Bajpai J, Barrios CH, Bergh J, Bergsten-Nordström E, Biganzoli L. 6th and 7th International consensus guidelines for the management of advanced breast cancer (ABC guidelines 6 and 7). The breast. 2024 Aug 1;76:103756.
6-Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). European heart journal. 2022 Nov 1;43(41):4229-361.
Approved by Egyptian Drug Authority: HF0082OA4733/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0424OA4807/102025 14/10/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
