
Guidelines
NCCN 2025 Guidelines and where Kisqali is positioned amongst the lines of treatment in mBC
Image

| National Comprehensive Cancer Network® (NCCN®) now differentiates ribociclib (KISQALI®) as the only Category 1 Preferred first-line treatment option in combination with an AI for patients with HR+/HER2- mBC.4 |
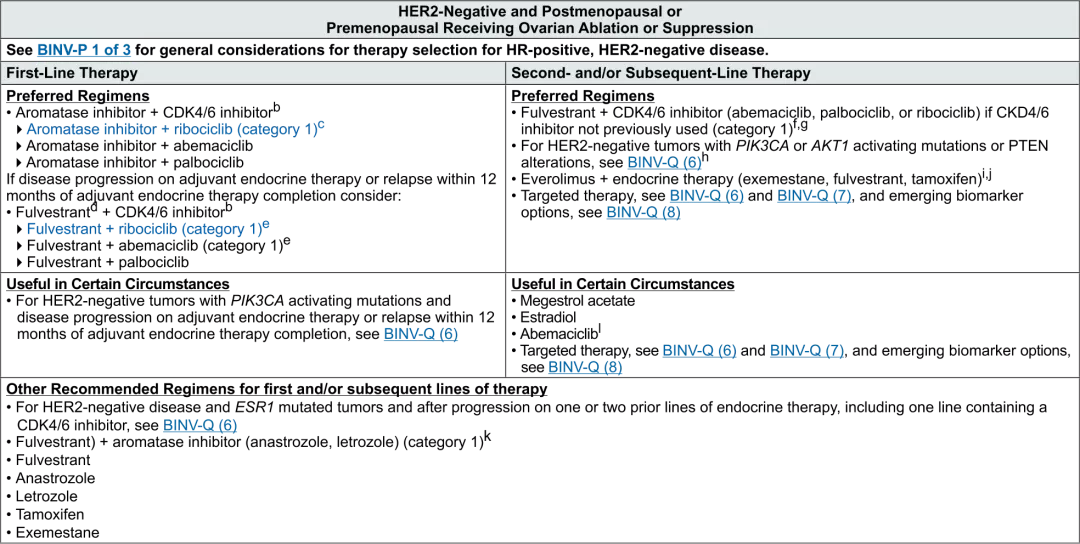

a Baseline assessment of bone density recommended for patients receiving an aromatase inhibitor who are at risk of osteoporosis (eg, age >65, family history, chronic
steroids).
b There is controversy on the choice of CDK4/6 inhibitor as there are no head to head comparisons between the agents and there are some differences in the study populations in the phase 3 randomized studies.
c In phase 3 randomized controlled trials, ribociclib + endocrine therapy have shown OS benefit in the first-line setting.
d Consider for disease progression on adjuvant endocrine therapy or with early disease relapse within 12 months of adjuvant endocrine therapy completion
e In phase 3 randomized controlled trials, fulvestrant + ribociclib or abemaciclib has shown OS benefit in the first-line setting
f In phase 3 randomized controlled trials, fulvestrant in combination with a CDK4/6 inhibitor (abemaciclib, palbociclib, and ribociclib) has shown OS benefit in the secondline setting.
g If there is disease progression while on palbociclib, there are limited phase II data to support the use of ribociclib in the second line setting.
h If there is progression while on a PI3K inhibitor, there are limited data to support another line of therapy with a PI3K-pathway inhibitor-containing regimen.
i If there is disease progression while on an everolimus-containing regimen, there are no data to support an additional line of therapy with another everolimus regimen.
j A combination of exemestane with everolimus can be considered for patients who meet the eligibility criteria for BOLERO-2 (progressed within 12 mo or on nonsteroidal aromatase inhibitor).
k A single study (S0226) in patients with HR-positive breast cancer and no prior chemotherapy, biological therapy, or endocrine therapy for metastatic disease demonstrated that the addition of fulvestrant to anastrozole resulted in prolongation of time to progression and OS. Subset analysis suggested that patients without prior adjuvant tamoxifen and more than 10 years since diagnosis experienced the greatest benefit. Two studies with similar design (FACT and SOFEA) demonstrated no advantage in time to progression with the addition of fulvestrant to anastrozole.
l Indicated after progression on prior endocrine therapy and prior chemotherapy in the metastatic setting.
KISQALI—Optimize outcomes with early monitoring5
Image

| DAILY DOSE: 600 mg (3 X 200 mg tablets taken at the same time)5With patient-friendly packaging
- Designed to keep things simple and encourage compliance |
Image

| FOOD RESTRICTIONS5Can be taken with or without food |
Image

| TABLET STRENGTH5200 mg tablets allow for easy dose adjustments
- No treatment disruptions or additional prescriptions needed when adjusting the dose mid-cycle.- Existing supply of KISQALI can be used to adjust the dose. |
A once-daily CDK4/6 inhibitor taken with or without food5
KISQALI—Specific Manageable AEs with clear dose modifications5
A CDK4/6 INHIBITOR THAT OFFERS ADJUSTABLE DOSING WITHOUT THE NEED FOR A NEW PRESCRIPTION MID-CYCLE5
Recommended dose | 600 mg/day | Image

| Image

|
1st reduction | 400 mg/day | Image

| Image

|
2nd reduction | 200 mg/day* | Image

| Image

|
* If further dose reduction below 200 mg/day is required, the treatment should be permanently discontinued.
Each film-coated tablet contains 200 mg of KISQALI5
Management of severe or intolerable adverse reactions (ARs) may require temporary dose interruption, reduction or discontinuation of KISQALI5
The clinical judgement of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment5
If dose reduction below 200 mg/day is required, discontinue treatment5
AEs: Adverse events
References
1.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, Campone M, Petrakova K, Winer EP, Janni W, Conte P. Overall survival with ribociclib plus letrozole in advanced breast cancer. New England Journal of Medicine. 2022 Mar 10;386(10):942-50.
2.Lu YS, Im SA, Colleoni M, Franke F, Bardia A, Cardoso F, Harbeck N, Hurvitz S, Chow L, Sohn J, Lee KS. Updated Overall Survival of Ribociclib plus Endocrine Therapy versus Endocrine Therapy Alone in Pre-and Perimenopausal Patients with HR+/HER2− Advanced Breast Cancer in MONALEESA-7: A Phase III Randomized Clinical Trial Updated Overall Survival Analysis of the MONALEESA-7 Trial. Clinical Cancer Research. 2022 Mar 1:OF1-9.
3.Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S, Petrakova K, Bianchi GV, Martín M, Nusch A, Sonke GS. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Annals of Oncology. 2021 Aug 1;32(8):1015-24.
4-National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Breast Cancer 2025. available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf . last accessed 3/2/2025
5-KISQALI. Summary of Products Characteristics (SMPC). available at: https://www.ema.europa.eu/en/documents/product-information/kisqali-epar-product-information_en.pdf last accessed 16/6/2025.
Approved by Egyptian Drug Authority: HF0082OA4733/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0424OA4807/102025 14/10/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
