
Efficacy - Major trial results
The most important treatment goal for patients diagnosed with HR+/HER2-ve advanced Breast Cancer: Living Longer1 , Let’s examine how Kisqali reaches this goal in different patient profiles
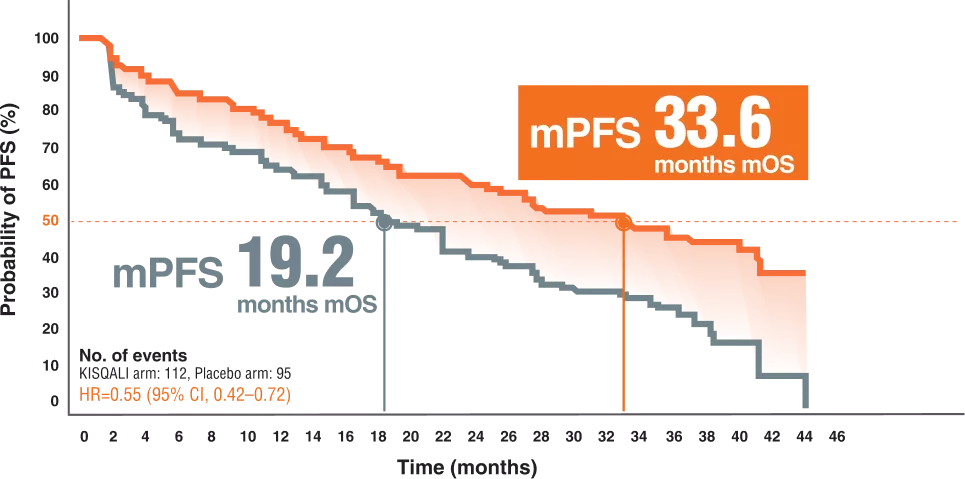
Post menopausal efficacy Monaleesa-2 ( Kisqali + Letrozole ) OS & PFS
MONALEESA-2 was a phase III, first-line trial in HR+/HER2– patients2
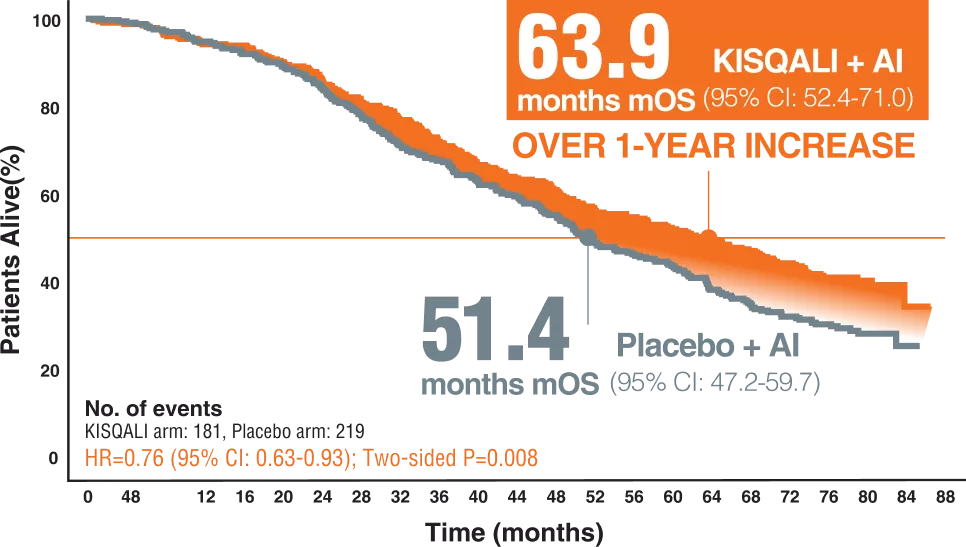
OVERALL SURVIVAL | KISQALI + AI (LET)2
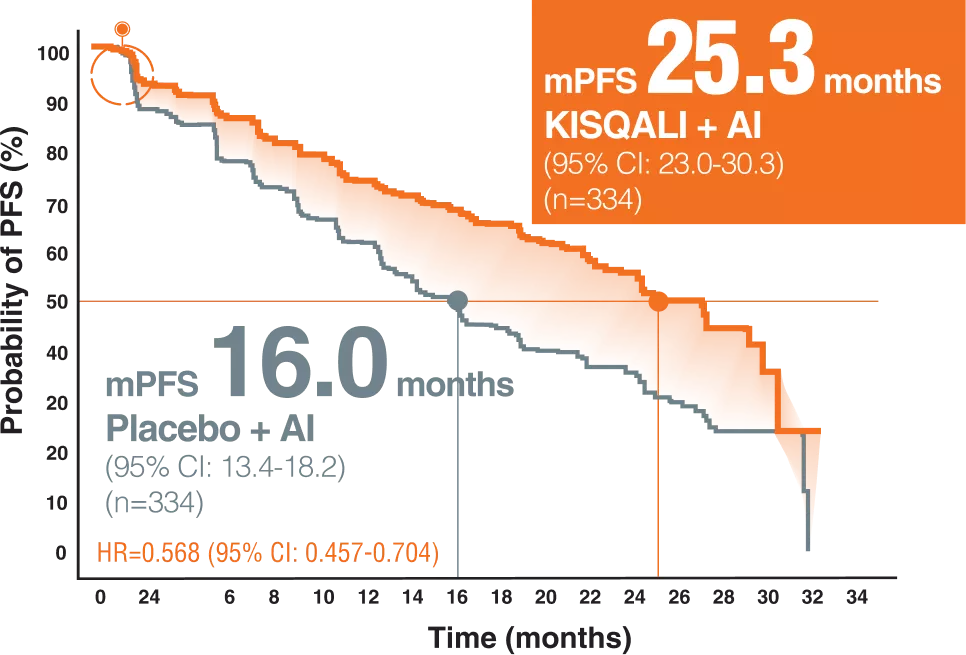
PFS PER INVESTIGATOR ASSESSMENT3
Study Design:
The MONALEESA-2 trial is a phase 3 trial evaluating the efficacy and safety of ribociclib in combination with letrozole as the first line of any treatment in postmenopausal patients with HR-positive, HER2-negative advanced breast cancer.2
A total of 668 patients were randomly assigned to receive KISQALI + AI or placebo + AI (KISQALI 600 mg or placebo once daily (3 weeks on, 1 week off) in combination with an AI (2.5 mg) once daily)2
Postmenopausal women with locally confirmed, HR-positive, HER2-negative recurrent or metastatic breast cancer who had not received previoussystemic therapy for advanced disease were eligible.2
Important treatment considerations:
59% visceral metastases, 34.1% had ≥ 3 metastatic sites.4
MONALEESA-2 trial Subgroup Analysis
Post menopausal efficacy Monaleesa-3 ( Kisqali + Fulverstrant ) OS & PFS
KISQALI-,a CDK4/6 inhibitor with FIRST-LINE evidence across PHASE III TRIALS, MONALEESA-3 Trial5
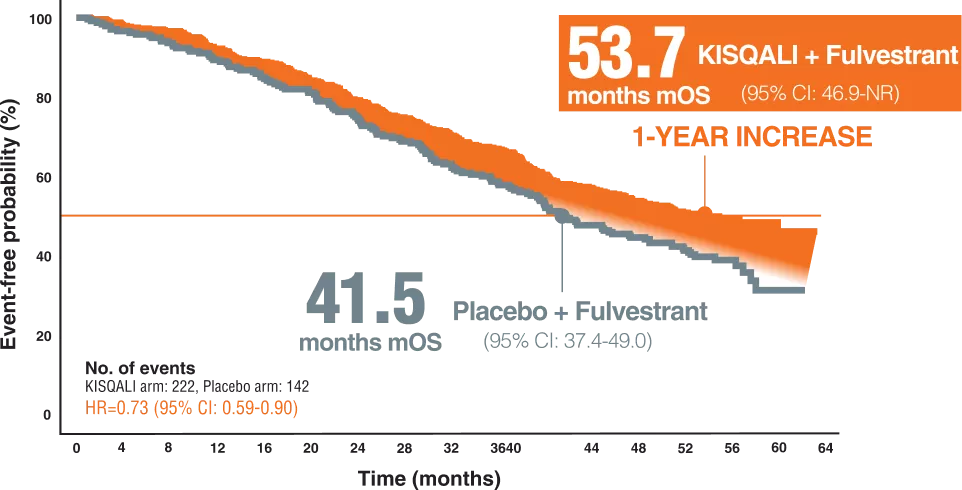
OVERALL SURVIVAL6
PFS PER INVESTIGATOR ASSESSMENT7
Study Design:
The MONALEESA-3 trial is phase III, double-blind, placebo-controlled international study,evaluating ribociclib plus fulvestrant in patients with HR-positive/ HER2-negative advanced breast cancer who were treatment naïve in the advanced setting or had received up to one line of prior endocrine therapy for advanced disease.5
A total of 726 patients were randomly assigned to were randomly assigned at a 2:1 ratio to receive KISQALI (600 mg orally per day; 3 weeks on, 1 week off) plus Fulvestrant (500 mg intramuscularly on day 1 of each 28-day cycle, with an additional dose on day 15 of cycle 1) or placebo plus Fulvestrant.5
A total of 354 patients were treatment na¨ıve for advanced disease, and 372 patients had received up to one line of prior endocrine therapy for advanced disease.5
No or only 1 line of prior ET for advanced disease5
Important treatment considerations:
60.5% visceral metastases, 48% liver and/or lung metastases.5
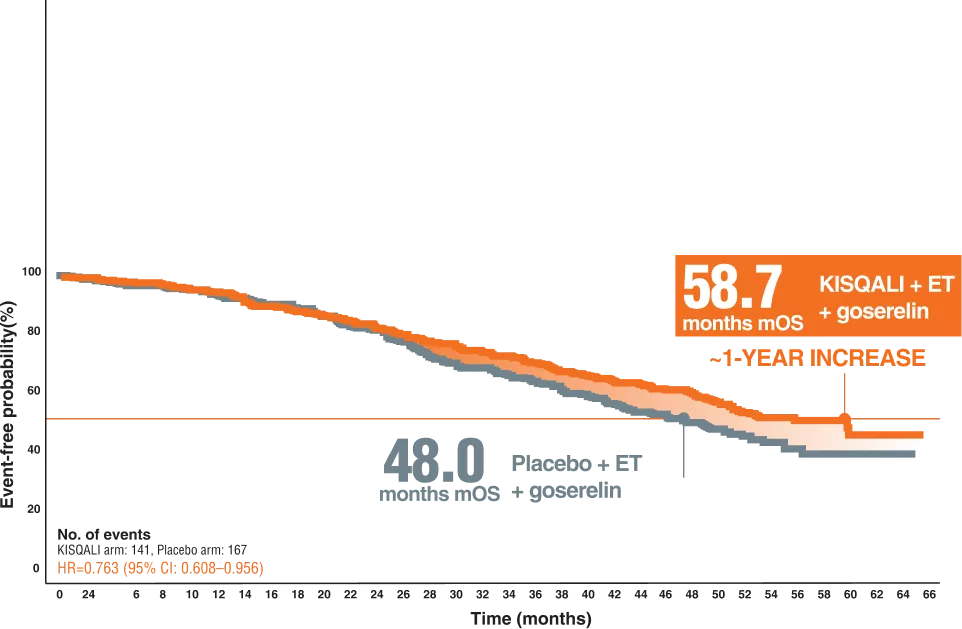
Pre-menopausal efficacy ( Monaleesa-7 ( Kisqali + AI + Goserelin ) OS & PFS
KISQALI-,a CDK4/6 inhibitor with FIRST-LINE evidence across PHASE III TRIALS, MONALEESA-7 Trial8
OVERALL SURVIVAL | KISQALI + ET + GOSERELIN9

PFS PER INVESTIGATOR ASSESSMENT10
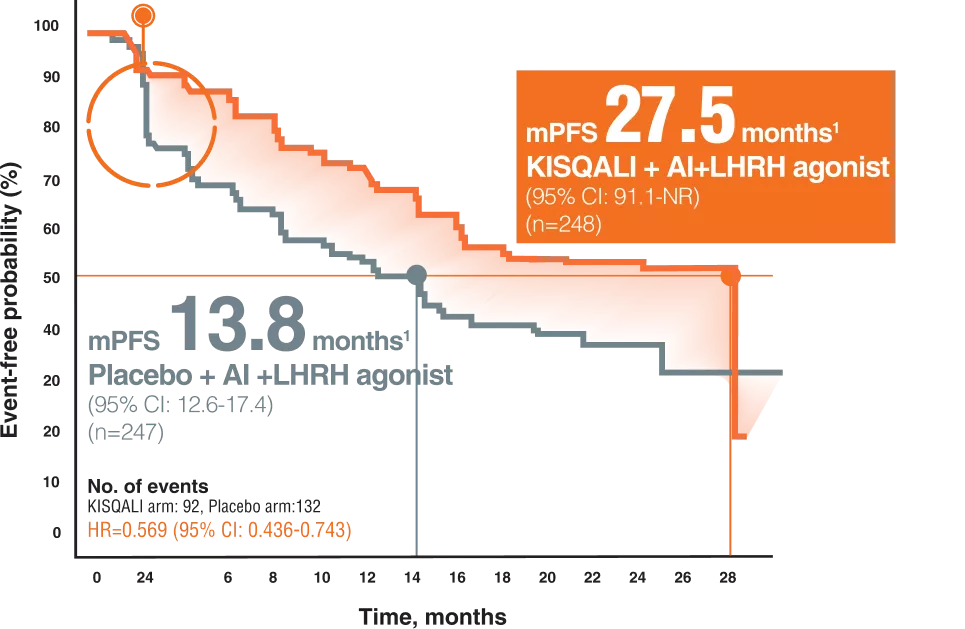
*Results reported at 8 weeks were not prespecified and are observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error.
Study Design:
The MONALEESA-7 trial was The FIRST DEDICATED PREMENOPAUSAL trial9
a randomized, double-blind, placebo-controlled, phase III trial that was conducted in 188 centers in 30 countries, and patients were randomized 1:1 to KISQALI (orally, 600 mg/day on 3-weeks-on, 1-week-off schedule) or matching placebo. Both groups received goserelin (subcutaneously 3.6 mg on day 1 of each 28-day cycle) and simultaneously also received either an NSAI (letrozole 2.5mgor anastrozole 1 mg, both orally, daily) or tamoxifen (20 mg daily).9
495 first-line patients8
Important treatment considerations:
27.6% of patients younger than 40,57% visceral metastases8
Pre-menopausal efficacy (RIGHT Choice ( Kisqali + ET + Goserelin or combination CT of investigator’s choice ) TTF & PFS
KISQALI,a CDK4/6 inhibitor with FIRST-LINE evidence across TRIALS, RIGHT Choice Trial10
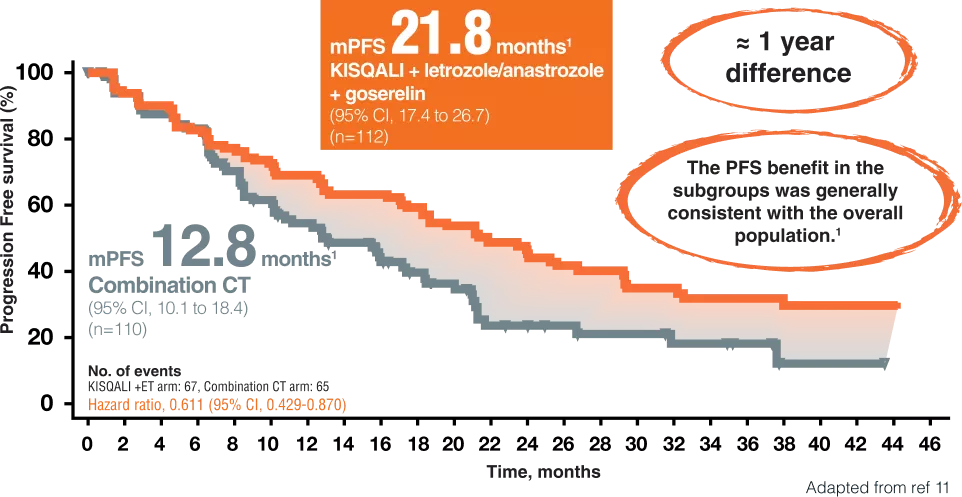
PFS PER INVESTIGATOR ASSESSMENT 11
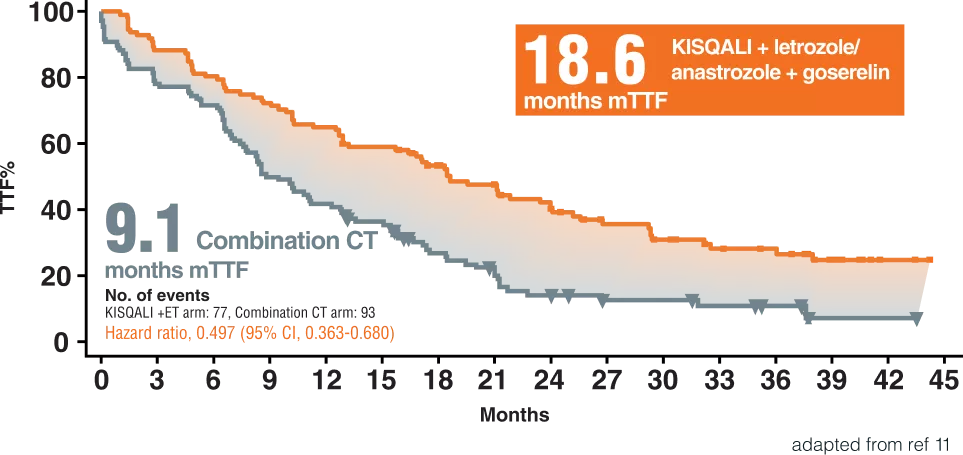
Time to Treatment Failure11

Study Design:
RIGHT Choice Trial was a dedicated premenopausal trial11
• 222 first-line patients11
• Randomized 1:1 to receive KISQALI first-line, letrozole/anastrozole and goserelin or investigator’s choice of combination Chemotherapy (docetaxel + capecitabine, paclitaxel + gemcitabine, orcapecitabine + vinorelbine). 11
Important treatment considerations:
Overall, (47.7%) had investigator assessed visceral crisis:In total, (67.6%) had symptomatic visceral metastases,(14.0%) had symptomatic non-visceral metastases.11
We report the final analysis of the phase II RIGHT Choice trial of first-line ribociclib plus ET versus combination CT in premenopausal women with clinically aggressive HR1/HER2-ABC, including investigator-assessed visceral crisis. 11

The data show:
- PFS superiority with ribociclib plus ET over combination CT, with similar response rates.
- Lower symptomatic AE rates.
- Fewer discontinuations because of treatment-related AEs.

HR+/HER2-: Hormone Receptor–Positive/Human Epidermal Growth Receptor 2–Negative, AI: Aromatase Inhibitor, ET: Endocrine Therapy; CI, confidence interval; HR, hazard ratio; LET, letrozole; mOS, median overall survival; mPFS, median progression-free survival, aBC:advanced Breast Cancer; HR, hazard ratio; ITT, intention to treat; LET, letrozole; mOS, median overall survival; OS, overall survival; PBO, placebo; RIB, ribociclib; NR: not reached.
References
Davie A, Carter GC, Rider A, Pike J, Lewis K, Bailey A, Price GL, Ringeisen F, Pivot X. Real-world patient-reported outcomes of women receiving initial endocrine-based therapy for HR+/HER2− advanced breast cancer in five European countries. BMC cancer. 2020 Dec;20(1):1-5.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, Campone M, Petrakova K, Winer EP, Janni W, Conte P. Overall survival with ribociclib plus letrozole in advanced breast cancer. New England Journal of Medicine. 2022 Mar 10;386(10):942-50.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Petrakova K, Blackwell KL, Winer EP, Janni W. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Annals of Oncology.
2018 Jul 1;29(7):1541-7.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, André F,Winer EP, Janni W. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. New England journal of medicine. 2016 Nov 3;375(18):1738-48.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín M,Nusch A. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. Journal of Clinical Oncology. 2018 Aug 20;36(24):2465-72.
Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S, Petrakova K, Bianchi GV, Martín M, Nusch A, Sonke GS. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall
survival. Annals of Oncology. 2021 Aug 1;32(8):1015-24.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín M, Nusch A. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. New England Journal of medicine. 2020 Feb 6;382(6):514-24.
Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, Hurvitz SA, Chow L, Sohn J, Lee KS, Campos-Gomez S.Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. The Lancet Oncology. 2018 Jul 1;19(7):904-15.
Lu YS, Im SA, Colleoni M, Franke F, Bardia A, Cardoso F, Harbeck N, Hurvitz S, Chow L, Sohn J, Lee KS. Updated Overall Survival of Ribociclib plus Endocrine Therapy versus Endocrine Therapy Alone in Pre-and Perimenopausal Patients with HR+/HER2− Advanced Breast Cancer in MONALEESA-7: A Phase III Randomized Clinical Trial Updated Overall Survival Analysis of the MONALEESA-7 Trial. Clinical Cancer Research. 2022 Mar 1:OF1-9.
KISQALI. Summary of Products Characteristics (SMPC). available at: https://www.ema.europa.eu/en/documents/
product-information/kisqali-epar-product-information_en.pdf . last accessed 16/6/2025Lu YS, Bin Mohd Mahidin EI, Azim H, Eralp Y, Yap YS, Im SA, Rihani J, Gokmen E, El Bastawisy A, Karadurmus N, Lim YN. Final results of RIGHT Choice: Ribociclib plus endocrine therapy vs combination chemotherapy in premenopausal women with clinically aggressive HR+/HER2− advanced breast cancer. Journal of Clinical Oncology. 2024 May 21:JCO-24.
Approved by Egyptian Drug Authority: HF0082OA4733/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0424OA4807/102025 14/10/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
