
Dosing & Duration
JAKAVI offers adjustable dosing to fit patient needs4
JAKAVI is a twice daily, oral treatment4
STARTING DOSE for GvHD4
Recommended starting dose for JAKAVI is | SVG
|

Dosing recommendations for patients with thrombocytopenia, neutropenia, or elevated total bilirubin in patients with graft-versus-host disease4
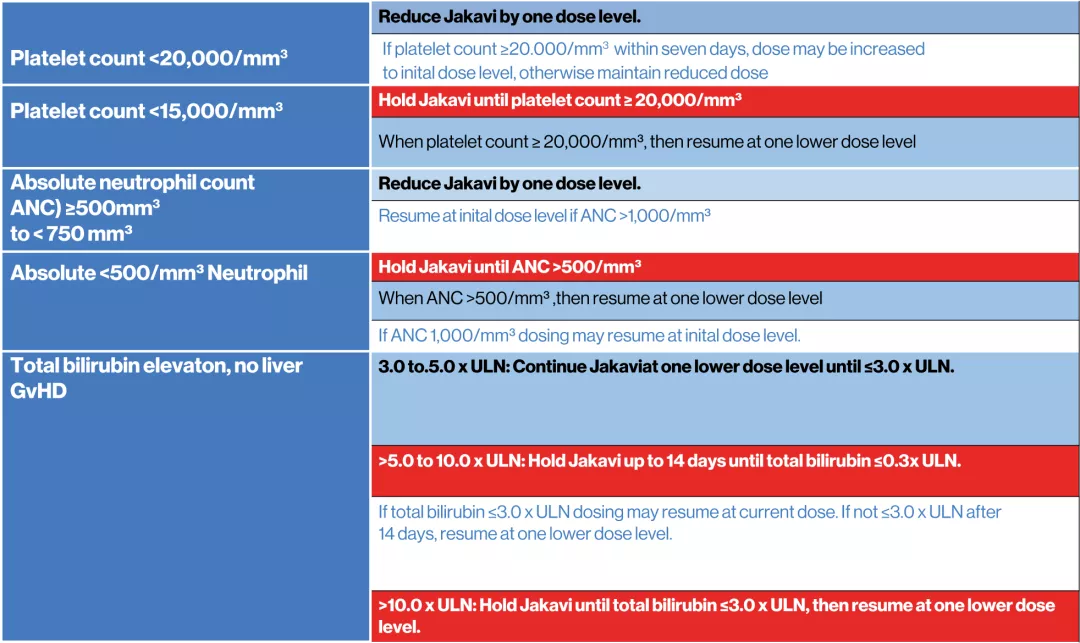
JAKAVI offers adjustable dosing to fit patient needs4
Long-term outcomes of JAKAVI in SR-GvHD through Real-world Evidence
Overall survival
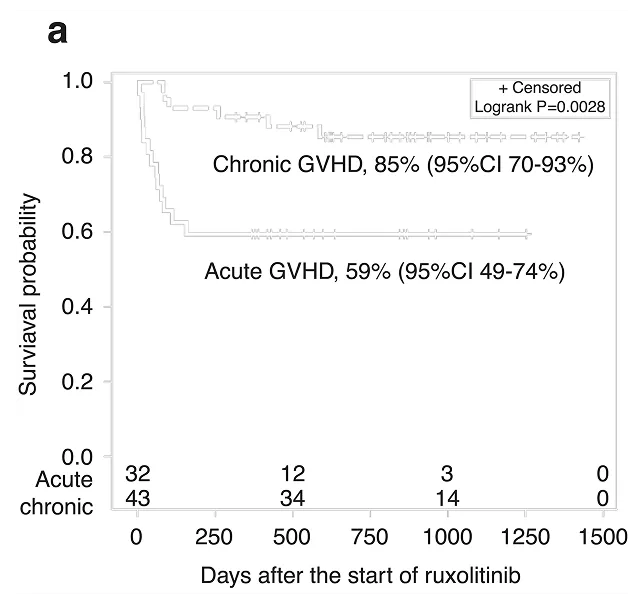
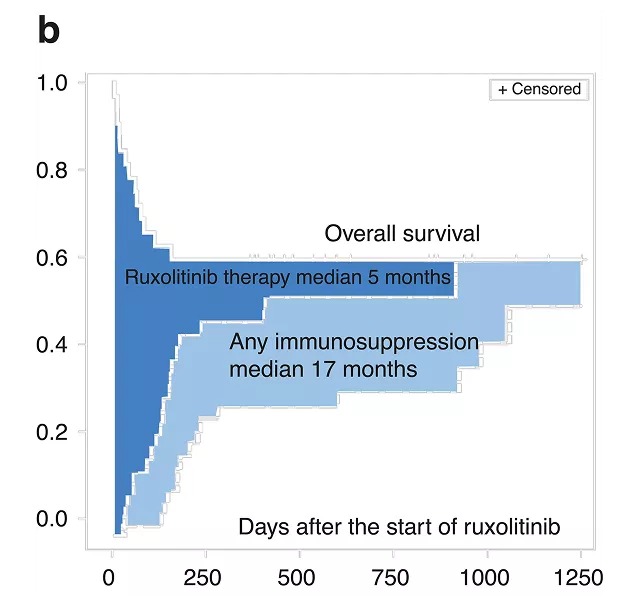
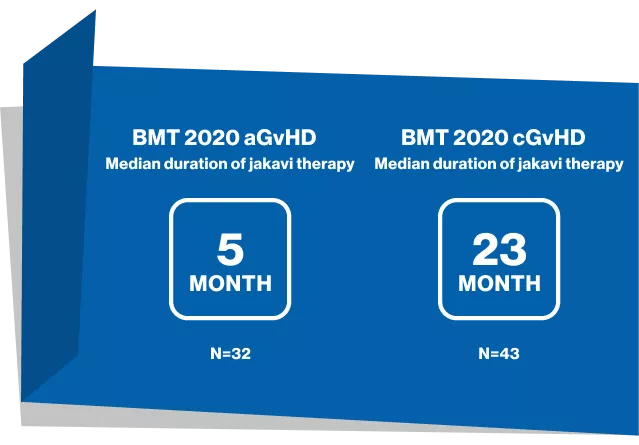
Duration of therapy in acute srGVHD
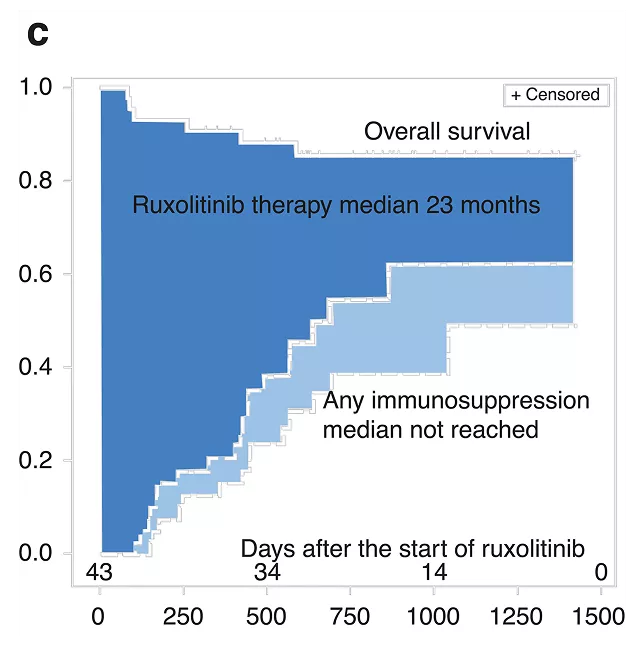
Duration of therapy in chronic srGVHD
EBMT, European Society for Blood and Marrow Transplantation; NCCN, National Comprehensive Cancer Network; GvHD, graft-versus-host disease
For JAKAVI -GvHD Abbreviated prescribing information
For JAKAVI -GvHD Abbreviated prescribing information
References
Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, Giebel S, Greinix H, Hazenberg MD, Kröger N, Mielke S. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. The Lancet Haematology. 2024 Feb 1;11(2):e147-59. available at https://www.thelancet.com/journals/lanhae/article/PIIS2352
3026(23)00342-3/abstract last accessed 12/8/2024(NCCN Guidelines®) for Hematopoietic Cell Transplantation (HCT). V.3.2023 available at: https://www.nccn.org/professionals/physician_gls/pdf/hct.pdf
Last accessed 26/3/2024Summary of Product Characteristics (SmPC).Jakavi Tablets.Available at
https://www.ema.europa.eu/en/documents/product-information/jakavi-epar-product-information_en.pdf
Last Accessed 26/3/2024Jakavi Egyptian Drug Authority Approved Leaflet 18/3/2024
Moiseev IS, Morozova EV, Bykova TA, Paina OV, Smirnova AG, Dotsenko AA, Borzenkova ES, Galimov AN, Gudognikova YV, Ekushov KA, Kozhokar PV. Long-term outcomes of ruxolitinib therapy in steroid-refractory graft-versus-host disease in children and adults. Bone Marrow Transplantation.
2020 Jul;55(7):1379-87
Approved by Egyptian Drug Authority: HF0424OA4703/102025. Invalidation date: 17/12/2025.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0424OA4703/102025 17/12/2025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
