
Chronic
59-85% of patients with grade II-IV aGvHD progress to cGvHD4
Chronic GvHD (cGvHD)5
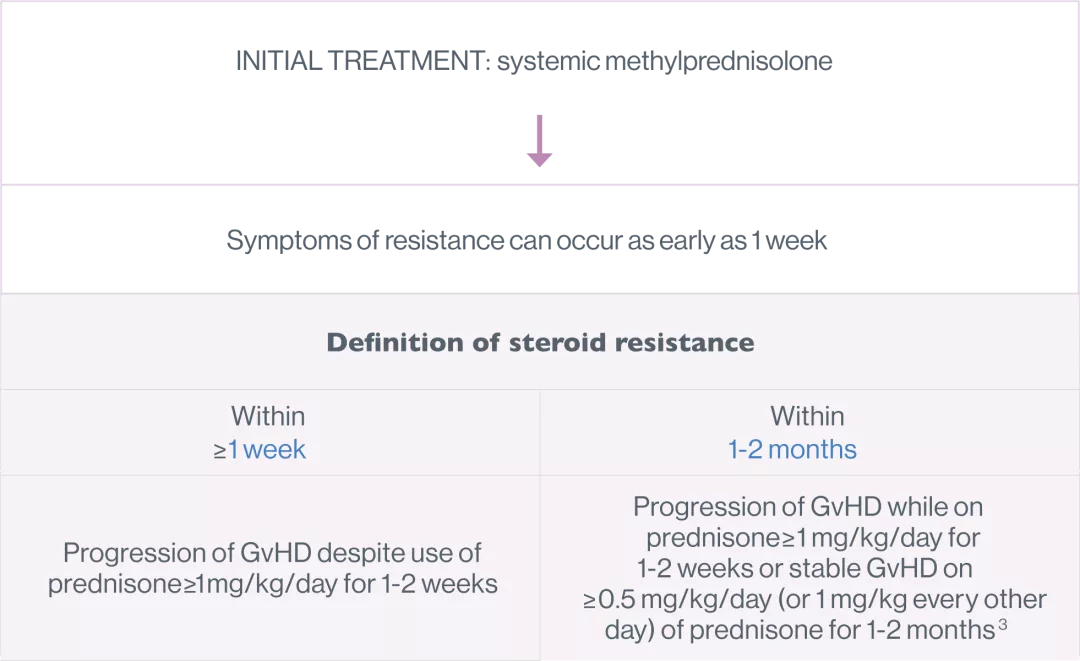
For steroid-resistant cGvHD, in patients 12 years or older6

In the REACH3 trial JAKAVI significantly improved ORR at week 24 compared with BAT arm7
OVERALL RESPONSE RATE7
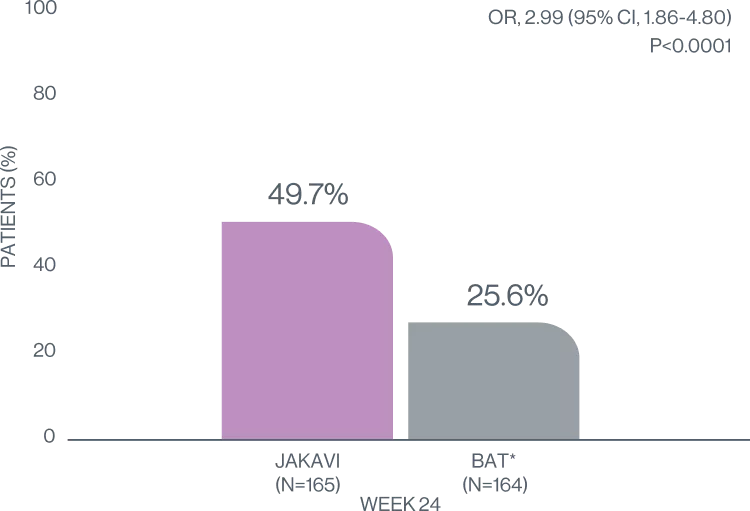
Treat your cGvHD patients with JAKAVI for nearly doubled ORR at week 247
In the groundbreaking REACH3 trial JAKAVI improved best overall response rate (ORR)3
BEST OVERALL RESPONSE*7
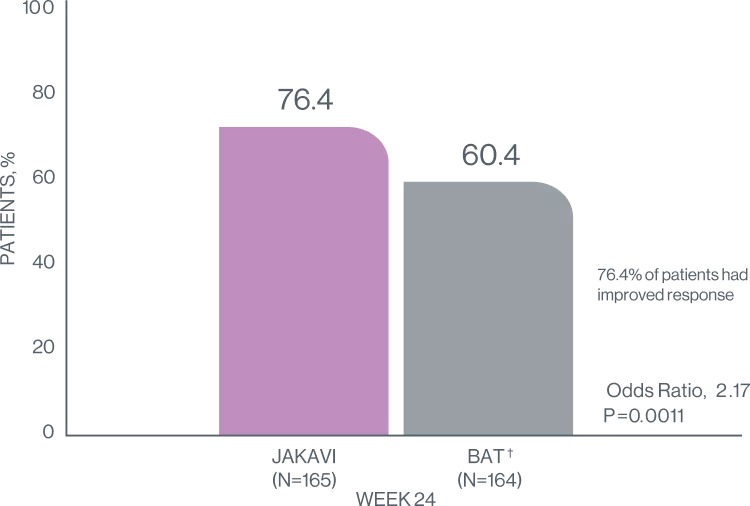
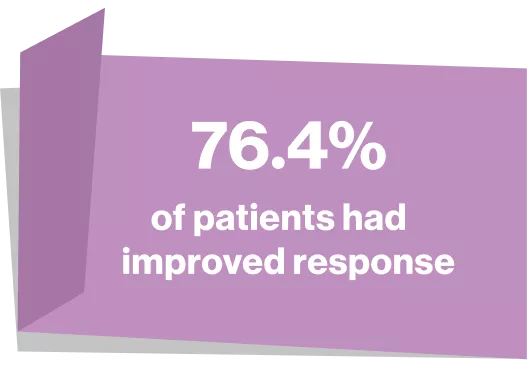
*Best overall response is defined as best response at any point during treatment. Better adherence to NIH consensus response criteria in REACH3 than in previous studies may have resulted in lower overall responses with control therapy and JAKAVI than have been reported previously7
Choosing JAKAVI provides improved response for cGvHD patients7
76.4 % in Jakavi group vs 60.4 in BAT group at week 247
JAKAVI provided superior organ response8
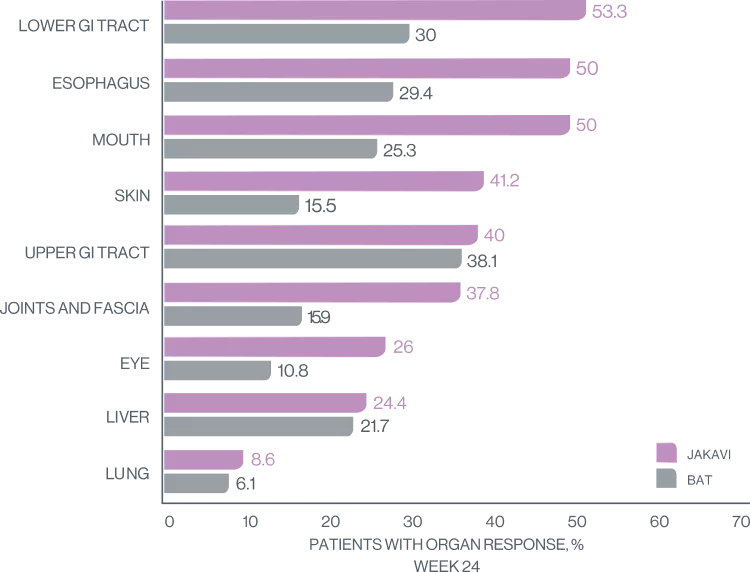
Treatment with JAKAVI demonstrates superior overall response and greater reduction in symptoms7
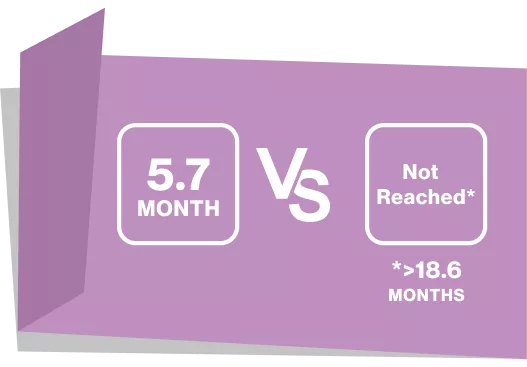
Understanding Progression-Free Survival (PFS)
“Failure-free survival, defined as Time to recurrence of underlying disease ; Start of new systemic treatment for chronic GVHD death whichever came first”7
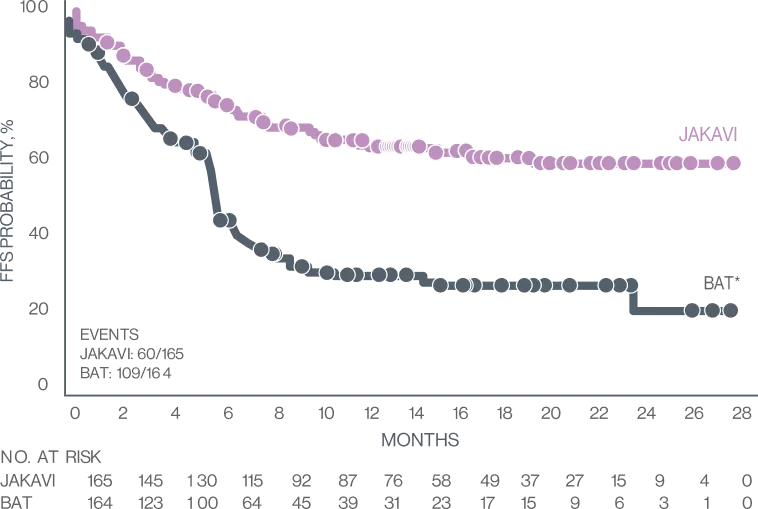
Failure-Free Survival7
KAPLAN-MEIER MEDIAN (JAKAVI VS BAT):
>18.6 MONTHS VS 5.7 MONTHS
HR, 0.37 (95% Cl, 0.27-0.51); P<0.001
*Best available therapy was chosen at the physician’s discretion from a list of 9 commonly used treatments: antithymocyte globulin, extracorporeal photopheresis, mesenchymal stromal cells, low-dose methotrexate,mycophenolate mofetil, mammalian target of rapamycin (mTOR) inhibitor (everolimus or sirolimus), etanercept, or iniximab3
Treating with JAKAVI provides longer failure -free survival for your cGvHD patients7
EBMT, European Society for Blood and Marrow Transplantation; NCCN, National Comprehensive Cancer Network; GvHD, graft-versus-host disease CR , Complete Response; PR, Partial Response; BAT, best available therapy
For JAKAVI -GvHD Abbreviated prescribing information
For JAKAVI -GvHD Abbreviated prescribing information
References
Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, Giebel S, Greinix H, Hazenberg MD, Kröger N, Mielke S. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. The Lancet Haematology. 2024 Feb 1;11(2):e147-59. available at https://www.thelancet.com/journals/lanhae/article/PIIS2352 3026(23)00342-3/abstract last accessed 12/8/2024
(NCCN Guidelines®) for Hematopoietic Cell Transplantation (HCT). V.3.2023 available at: https://www.nccn.org/professionals/physician_gls/pdf/hct.pdf Last accessed 26/3/2024
Summary of Product Characteristics (SmPC).Jakavi Tablets.Available at https://www.ema.europa.eu/en/documents/product-information/jakavi-epar-product-information_en.pdf Last Accessed 26/3/2024
Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone marrow transplantation. 2001 Jul;28(2):121-9.
DeFilipp Z, Couriel DR, Lazaryan A, Bhatt VR, Buxbaum NP, Alousi AM, Olivieri A, Pulanic D, Halter JP, Henderson LA, Zeiser R. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. The 2020 treatment of chronic GVHD report. Transplantation and cellular therapy. 2021 Sep 1;27(9):729-37. Available at: https://www.sciencedirect.com/science/article/pii/S2666636721008952. last accessed 12/8/2024
Jakavi Egyptian Drug Authority Approved Leaflet 18/3/2024
Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, Musso M, Giebel S, Uzay A, Langmuir P, Hollaender N. Ruxolitinib for glucocorticoid- refractory chronic graft-versushost disease. New England Journal of Medicine. 2021 Jul 15;385(3):228-38.
Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, Musso M, Giebel S, Uzay A, Langmuir P, Hollaender N. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. New England Journal of Medicine. 2021 Jul 15;385(3):228-38. (Supplemental Appendix). Available at: https://www.nejm.org/doi/suppl/10.1056/NEJMoa2033122/suppl_file/nejmoa2033122_appendix.pdf last accessed 12/8/2024
Approved by Egyptian Drug Authority: HF0424OA4703/102025. Invalidation date: 17/12/2025.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0424OA4703/102025 17/12/2025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
