
Acute
30% to 50% of allogeneic hematopoietic stem cell transplant (alloHSCT) patients develop acute GvHD (aGvHD)4
Acute GvHD (aGvHD)5
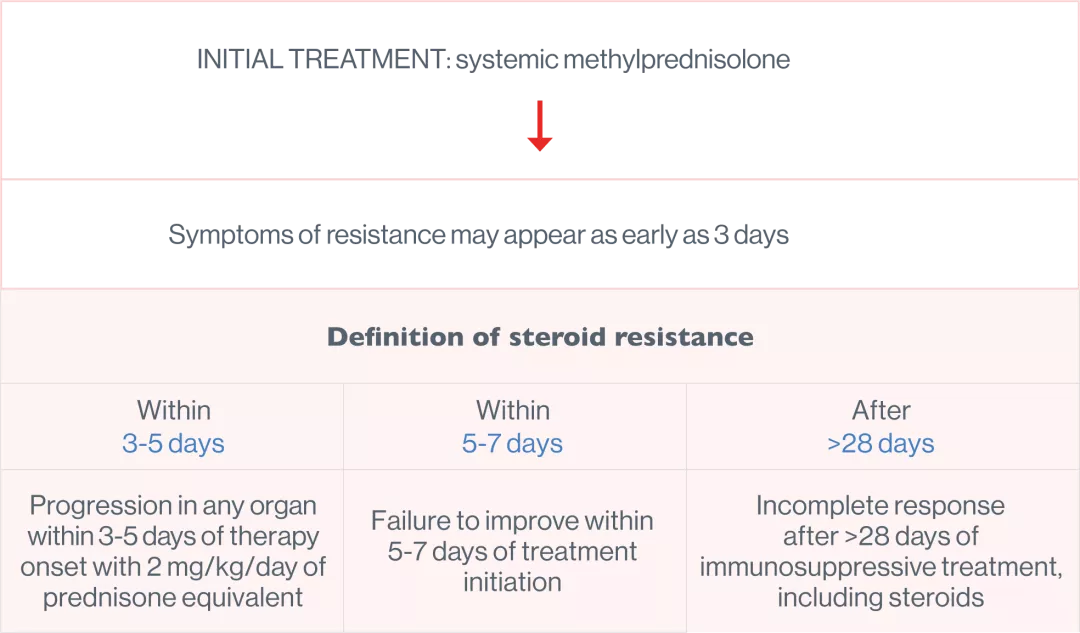
For steroid-resistant aGvHD, in patients 12 years or older6

In the groundbreaking REACH2 trial JAKAVI delivered a significant and sustained overall response rate (ORR)3
OVERALL RESPONSE 3
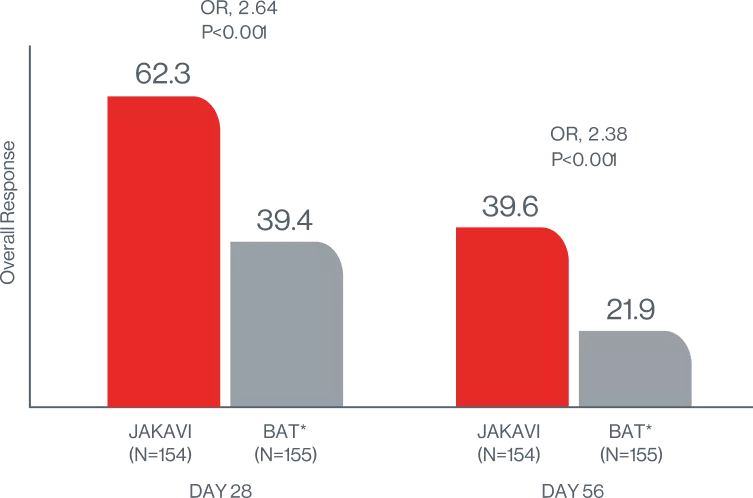

JAKAVI provided superior sustained failure-free survival (FFS)7
FAILURE-FREE SURVIVAL7
KAPLAN-MEIER MEDIAN (JAKAVI VS BAT):
5 months VS 1 month
HR, 0.46 (95% Cl, 0.35-0.60);
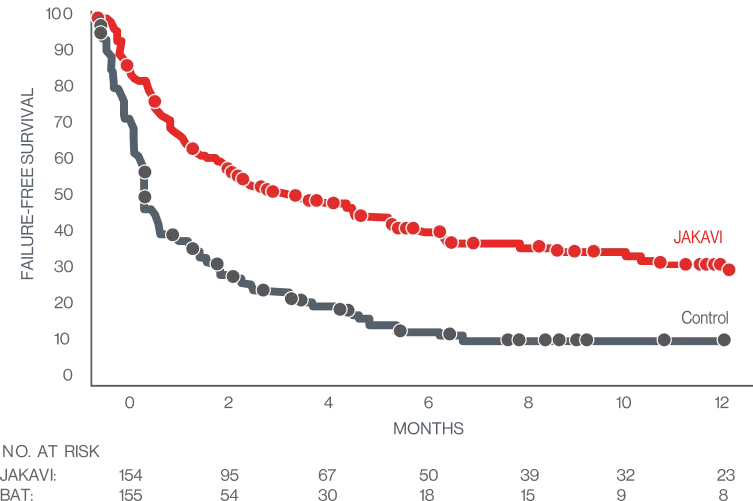

JAKAVI significantly improved overall survival (OS)7
OVERALL SURVIVAL8
KAPLAN-MEIER MEDIAN (JAKAVI VS BAT):
11.14 MONTHS VS 6.47 MONTHS
HR, 0.83 (95% C l, 0.60-1.15)

EBMT, European Society for Blood and Marrow Transplantation; NCCN, National Comprehensive Cancer Network; GvHD, graft-versus-host disease CR , Complete Response; PR, Partial Response; BAT, best available therapy
For JAKAVI -GvHD Abbreviated prescribing information
For JAKAVI -GvHD Abbreviated prescribing information
References
Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, Giebel S, Greinix H, Hazenberg MD, Kröger N, Mielke S. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. The Lancet Haematology. 2024 Feb 1;11(2):e147-59. available at https://www.thelancet.com/journals/lanhae/article/PIIS2352
3026(23)00342-3/abstract last accessed 12/8/2024(NCCN Guidelines®) for Hematopoietic Cell Transplantation (HCT). V.3.2023 available at: https://www.nccn.org/professionals/physician_gls/pdf/hct.pdf
Last accessed 26/3/2024Summary of Product Characteristics (SmPC).Jakavi Tablets.Available at
https://www.ema.europa.eu/en/documents/product-
information/jakavi-epar-product-information_en.pdf Last Accessed 26/3/2024Zeiser R, Blazar BR. Acute graft-versus-host disease—biologic process, prevention, and therapy. New England Journal of Medicine. 2017 Nov 30;377(22):2167-79
Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR. EBMT (European Society for Blood and Marrow Transplantation) Transplant Complications Working Party and the “EBMT− NIH (National Institutes of Health)− CIBMTR (Center for International Blood and Marrow Transplant Research) GvHD Task Force”. Bone Marrow Transplant. 2018;53(11):1401-15.
Jakavi Egyptian Drug Authority Approved Leaflet 18/3/20247.
Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, Szer J, Wagner EM, Zuckerman T, Mahuzier B, Xu J. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. New England Journal of Medicine. 2020 May 7;382(19):1800-10
Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, Szer J, Wagner EM, Zuckerman T, Mahuzier B, Xu J. Ruxolitinib for
glucocorticoid-refractory acute graft-versus-host disease. New England Journal of Medicine . 2020 May 7;382(19):1800-10(Supplementary Appendix) available at: https://www.nejm.org/doi/suppl/10.1056/NEJMoa1917635/suppl_file/nejmoa1917635_appendix.pdf
last accessed 14/8/2024
Approved by Egyptian Drug Authority: HF0424OA4703/102025. Invalidation date: 17/12/2025.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0424OA4703/102025 17/12/2025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
