
*Greater improvements at week 16 from baseline compared to placebo were demonstrated in health-related quality of life as measured by the Dermatology Life Quality Index. 1
The safety profile in the SUNSHINE and SUNRISE trials is consistent with that already reported, with no new or unexpected safety concerns detected, the study duration is 60 weeks2 | Image

|
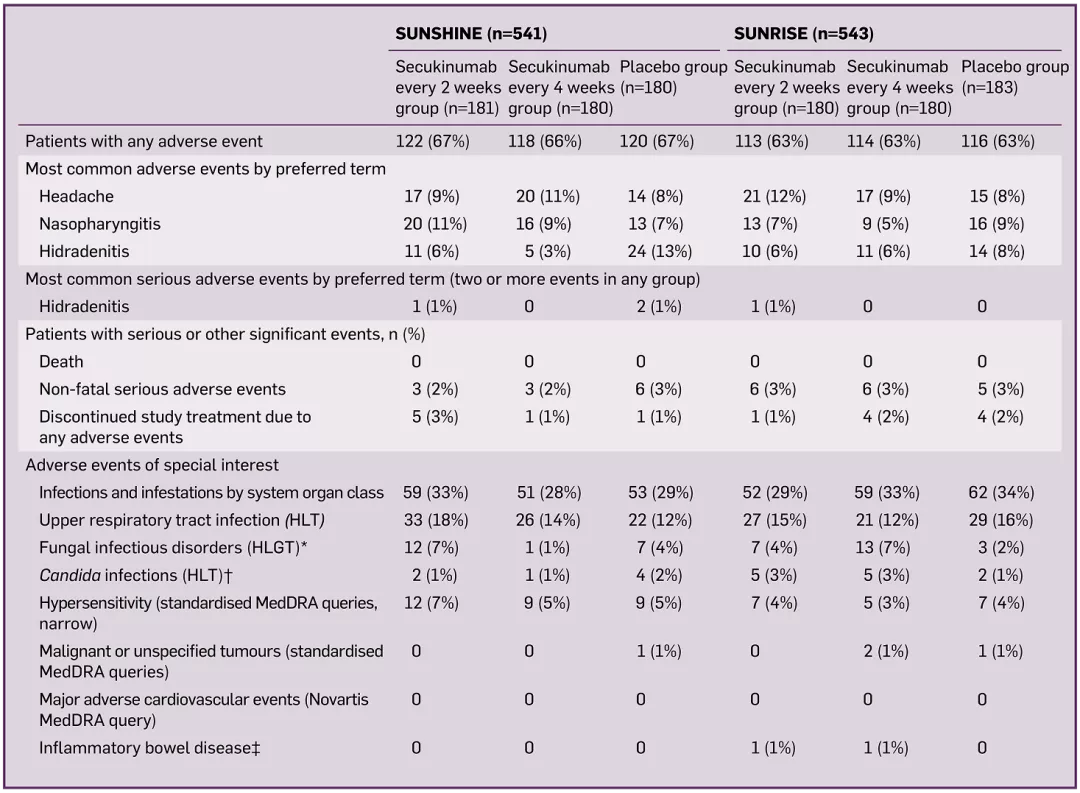
Adapted from Ref.2
HLGT=high-level group term; HLT=high-level term; MedDRA=medical dictionary for regulatory activities.
You can tailor the dosing based on your patients’ needs1 | Image

|
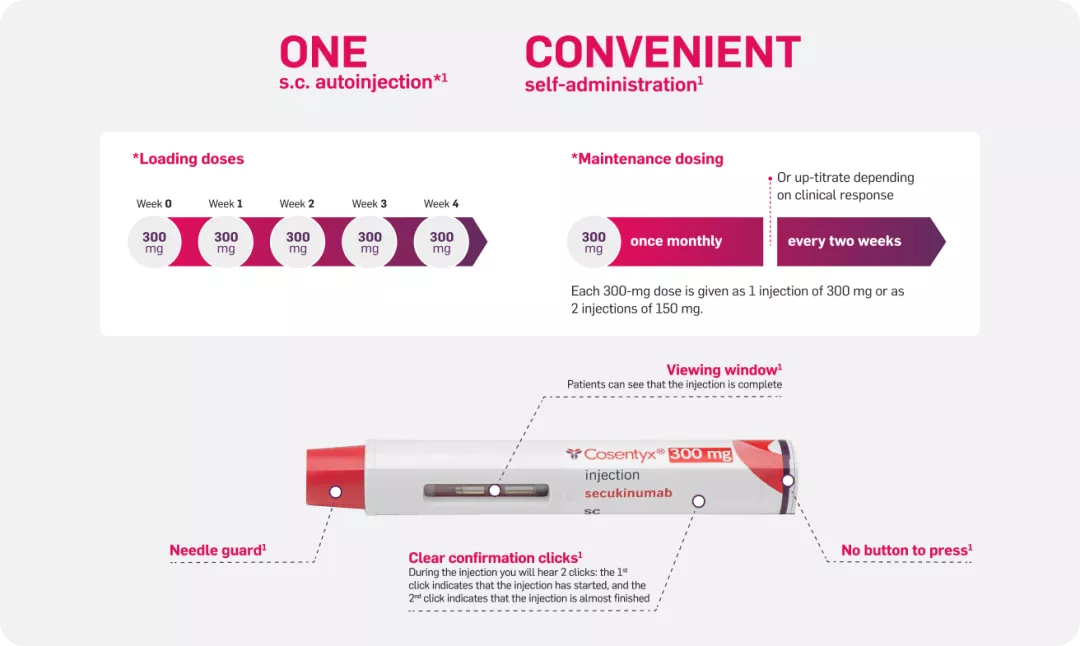
Cosentyx API
Cosentyx API
References
Cosentyx 150,300 mg Egyptian Drug Authority approved leaflet, Approval date: 23-03-2025.
Kimball AB, Jemec GB, Alavi A, Reguiai Z, Gottlieb AB, Bechara FG, Paul C, Bourboulis EJ, Villani AP, Schwinn A, Ruëff F. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): Week 16 and week 52 results of twoidentical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. The Lancet. 2023 Mar 4;401(10378):747-61.
Approved by Egyptian Drug Authority: BF0424OA4706/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
BF0424OA4706/082025 28/08/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
