
*Greater improvements at week 16 from baseline compared to placebo were demonstrated in health-related quality of life as measured by the Dermatology Life Quality Index. 1
For adults patients with active moderate to severe hidradenitis suppurativa (HS) (acne inversa) with an inadequate response to conventional systemic HS therapy1 | Image

|
Hurley staging severity scoring3

Stage 1
Solitary or multiple, isolated abscess formation without scarring or sinus tracts.3
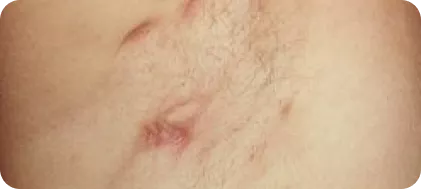
Stage 2
Recurrent abscesses, single or multiple widely separated lesions, with sinus tract formation.3

Stage 3
Diffuse or broad involvement, with multiple interconnected sinus tracts and abscesses.3
The SUNSHINE and SUNRISE Phase 3 Clinical Study Program
The largest trials ever done in hidradenitis suppurativa and the first
reporting efficacy and safety results up to 52 weeks of treatment 2
IL-17A expression is more highly expressed in HS compared to healthy skin,
149-fold increase in IL-17A mRNA expression in HS lesion versus control (p<0.01).*4
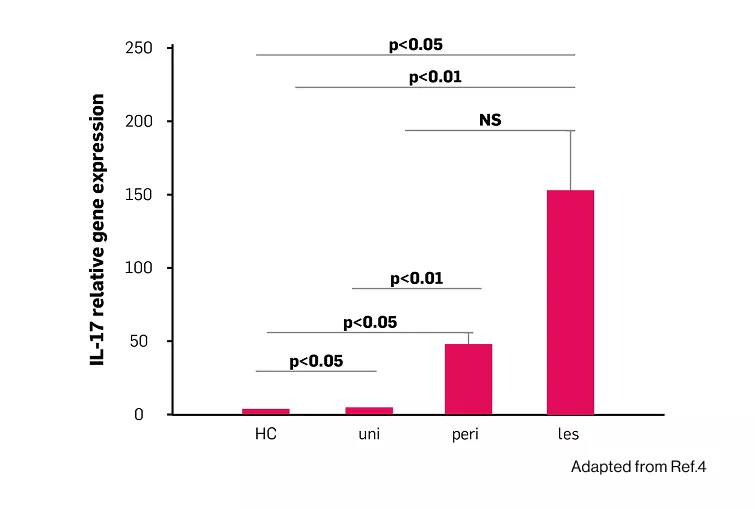
mRNA was isolated from uninvolved ( n = 5), perilesional ( n = 6) and lesional ( n = 11) HS skin and from healthy control skin (n = 5). The relative mRNA expression of IL17A was determined by reverse transcriptase polymerase chain reaction. 4
Study Design:
Three 6-mm punch biopsies were obtained from 44 patients with HS from (i) the leading edge of an active nodule, (ii) normal-appearing perilesional skin 2 cm from the active lesion and (iii) uninvolved skin 10 cm from the lesion. One 6-mm punch biopsy was obtained from healthy hospital-based volunteers or from healthy women undergoing breast-reduction surgery for cosmetic reasons (n = 10). The expression of various cytokines was determined by enzyme-linked immunosorbent assay, flow cytometry and real-time polymerase chain reaction. The trial investigated the skin of patients with HS for immunological dysregulation specifically by determining the levels of mRNA of several cytokines and by assessing which cells were producing these cytokines.4
IL= interleukin; HS= hidradenitis suppurativa; mRNA= messenger ribonucleic acid; HC= healthy control; NS= not significant; uni= uninvolved; peri= perilesional; les= lesional.
Cosentyx blocks IL-17A, a key cytokine responsible for inflammation that underlies axSpA, PsA, PsO, and HS1
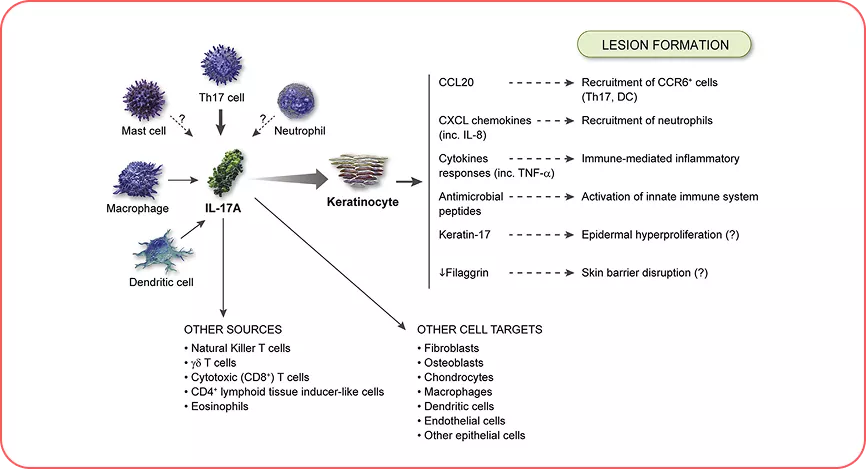
Adapted from Ref.5
Cellular sources and targets in psoriasis. T-helper (Th)17 cells are the key cellular sources, and keratinocytes are the key cellular target of interleukin (IL)-17A. Neutrophils stain positive for IL-17, but production of the protein has yet to be confirmed. Similarly, some evidence suggests that mast cells produce IL-17A, but further investigation is required.5
IL=interleukin; axSpA=axial spondyloarthritis; PsA=psoriatic arthritis; PsO=plaque arthritis; HS=hidradenitis suppurativa.
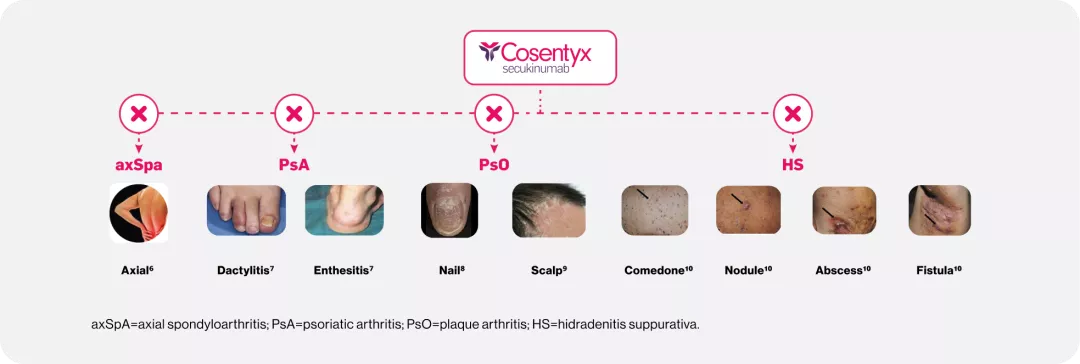
Increased levels of IL-17A are found in the affected tissues of patients with axSpA, PsA, PsO, HS1
Image

| Across 8 indications1 |








Cosentyx API
Cosentyx API
References
Cosentyx 150,300 mg Egyptian Drug Authority approved leaflet, Approval date: 23-03-2025.
Kimball AB, Jemec GB, Alavi A, Reguiai Z, Gottlieb AB, Bechara FG, Paul C, Bourboulis EJ, Villani AP, Schwinn A, Ruëff F. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): Week 16 and week 52 results of twoidentical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. The Lancet. 2023 Mar 4;401(10378):747-61.
Hidradenitis severity assessment. DermNet. Available at: https://dermnetnz.org/topics/hidradenitis-suppurativaseverityassessment; Last accessed: 19-06-2025.
Kelly G, Hughes R, McGarry TV, Van Den Born M, Adamzik K, Fitzgerald R, Lawlor C, Tobin AM, Sweeney CM, Kirby B. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. British Journal of Dermatology. 2015 Dec 1;173(6):1431-9.
Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. Journal of the American Academy of Dermatology. 2014 Jul 1;71(1):141-50.
What Is Axial Spondyloarthritis_ Symptoms, Causes, Diagnosis. Creakyjoints organization. Available at:https://creakyjoints.org/about-arthritis/axial-spondyloarthritis/axspa-overview/what-is-axial-spondyloarthritis/; last accessed :19-06-2025.
Bagel J, Schwartzman S. Enthesitis and dactylitis in psoriatic disease: a guide for dermatologists. American Journal of clinical dermatology. 2018 Dec;19(6):839-52.
What is nail psoriasis, and how can I treat it. American Academy of Dermatology. Available at: https://www.aad.org/public/diseases/psoriasis/treatment/genitals/nails; last accessed: 19-06-2025.
Scalp Psoriasis. Symptoms, Plaque, Causes & Treatment. Available at: https://my.clevelandclinic.org/health/diseases/22828-scalp-psoriasis; last accessed: 19-06-2025.
Hidradenitis suppurativa – Management, comorbidities and monitoring. RACGP. Available at: https://www.racgp.org.au/afp/2017/august/hidradenitis-suppurativa-management-comorbidities; Last accessed 19-06-2025.
Approved by Egyptian Drug Authority: BF0424OA4706/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
BF0424OA4706/082025 28/08/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |



