
*Greater improvements at week 16 from baseline compared to placebo were demonstrated in health-related quality of life as measured by the Dermatology Life Quality Index.1
In Sunshine and sunrise studies, the onset of action of Cosentyx 300mg occurred as early as week 2, the efficacy progressively increased to week 16 and was maintained up to week 522 | Image

|
The SUNSHINE and SUNRISE Phase 3 Clinical Study Program2
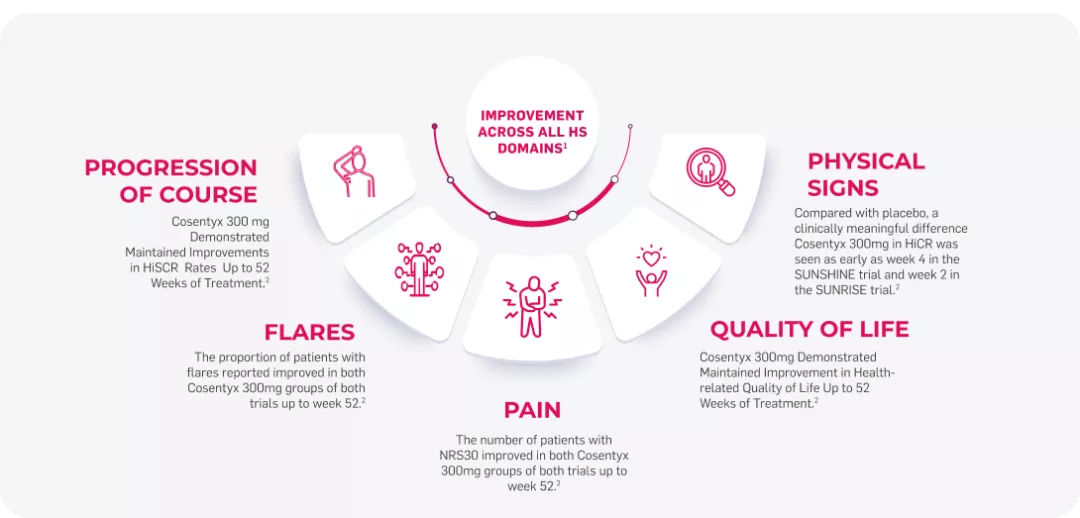
The largest trials ever done in hidradenitis suppurativa and the first reporting efficacy and safety results up to 52 weeks of treatment.2

SUNSHINE & SUNRISE both met primary endpoint (HiSCR)2
Image
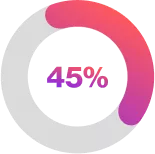
| of the Cosentyx 300mg Q2W group compared with 34% in the placebo group had a hidradenitis suppurativa clinical response (OR 1·8 [95% CI 1·1–2·7]; p=0·007) 2 |
Image
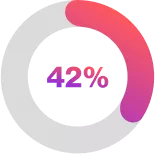
| of the Cosentyx 300mg Q4W group compared with 34% in the placebo group had a hidradenitis suppurativa clinical response (1·5 [95% CI 1·0–2·3]; p=0·042) 2 |
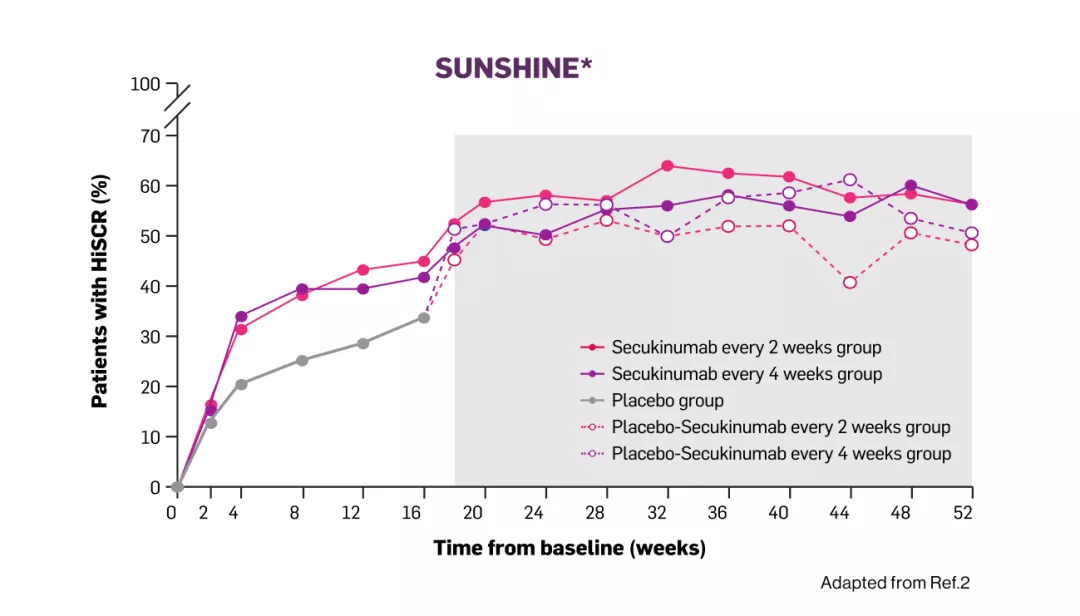
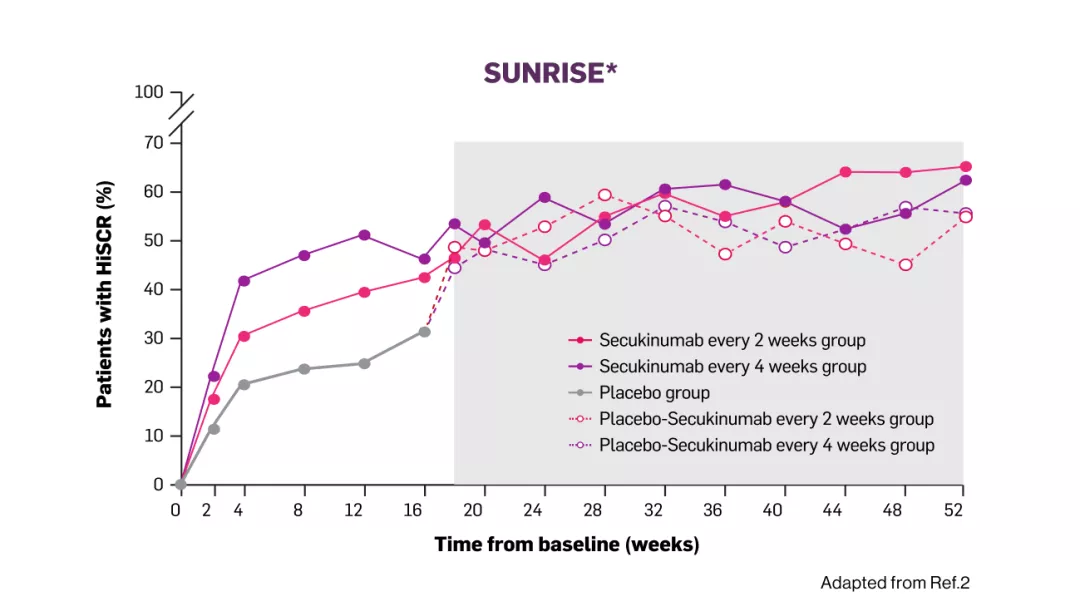
*Dashed lines represent patients switching from placebo at week 16. Grey box represents observed data.
HiSCR= hidradenitis suppurativa clinical response; Q2W= every two weeks; Q4W= every four weeks; CI= confidence intervals.
Image

| of the Cosentyx 300mg Q2W group compared with 31% in the placebo group had a hidradenitis suppurativa clinical response (1·6 [1·1–2·6]; p=0·015). 2 |
Image
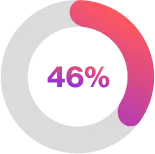
| of the Cosentyx 300mg Q4W group compared with 31% in the placebo group had a hidradenitis suppurativa clinical response (1·9 [1·2–3·0]; p=0·0022). 2 |
*Dashed lines represent patients switching from placebo at week 16. Grey box represents observed data.
HiSCR= hidradenitis suppurativa clinical response; Q2W= every two weeks; Q4W= every four weeks; CI= confidence intervals.
In both studies, the onset of action of Cosentyx 300mg occurred as early as week 2, the efficacy progressively increased to week 16 and was maintained up to week 521
Cosentyx API
Cosentyx API
References
Cosentyx 150,300 mg Egyptian Drug Authority approved leaflet, Approval date: 23-03-2025.
Kimball AB, Jemec GB, Alavi A, Reguiai Z, Gottlieb AB, Bechara FG, Paul C, Bourboulis EJ, Villani AP, Schwinn A, Ruëff F. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): Week 16 and week 52 results of twoidentical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. The Lancet. 2023 Mar 4;401(10378):747-61.
Approved by Egyptian Drug Authority: BF0424OA4706/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
BF0424OA4706/082025 28/08/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
