
LEQVIO® Core Data Sheet. Novartis Europharm Limited.
Raal FJ, Kallend D, Ray KK, et al; ORION-9 Investigators. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engel K Med. 2020;1:1-11. doi:10.1056/NEJMoa1913805
Data on file. ORION-9 (MDCO-PCS-17-03) Clinical Study Report. The Medicines Company; 2019. "to be provided if requested"
Data on file. ORION-10 (MDCO-PCS-17-04) Clinical Study Report. The Medicines Company; 2019. "to be provided if requested"
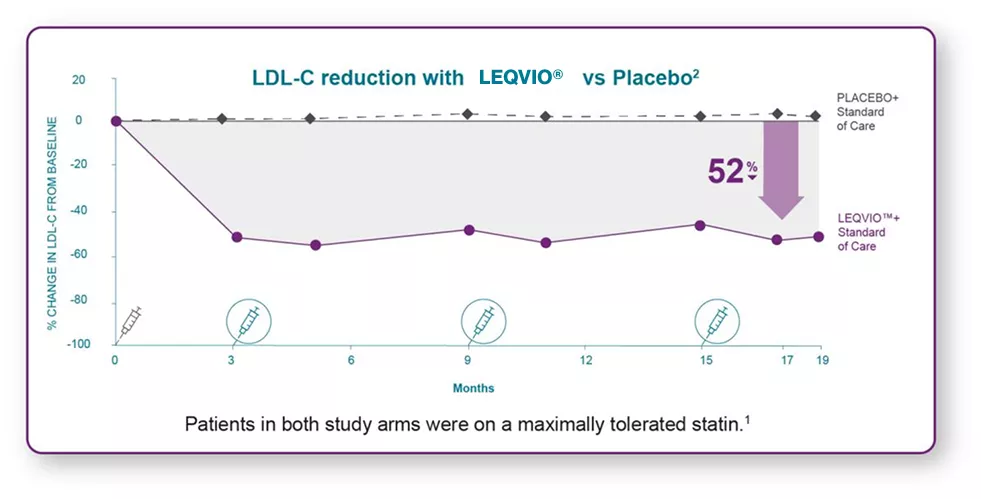
52% effective LDL-C reduction between-group difference at Month 17*
54% sustained LDL-C reduction between-group difference after Month 3 and up to Month 18†
LEQVIO® Core Data Sheet. Novartis Europharma Limited.
Ray KK, Wright RS, Kallend D, et al; ORION-10 and ORION-11 Investigators. Two phase 3 trials of Inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020,382(16):1507-1519. doi:10.1056/NEJMoa1912387.
Data on file. ORION-10 (MDCO-PCS-17-04) Clinical Study Report. The Medicines Company; 2019. "to be provided if requested"
Data on file. ORION-9 (MDCO-PCS-17-03) Clinical Study Report. The Medicines Company; 2019. "to be provided if requested"
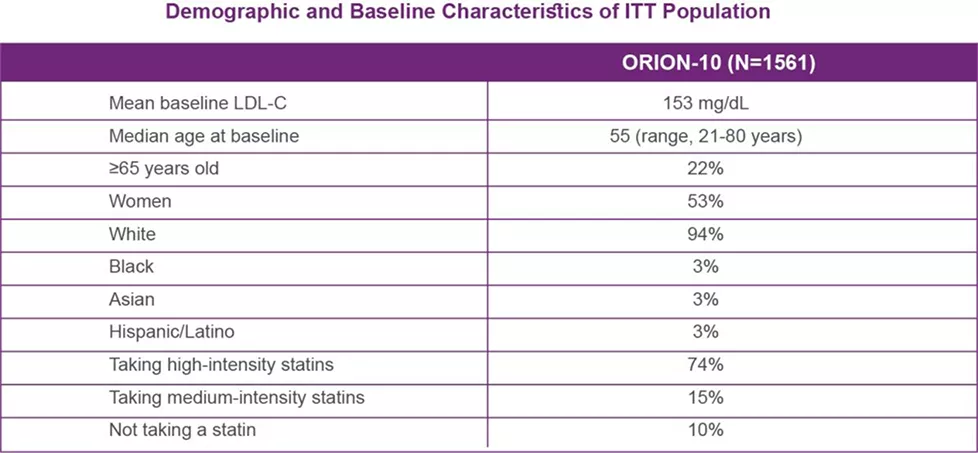
ORION-9: Efficacy in Heterozygous Familial Hypercholesterolemia
Study Design1
ORION-9 was a multicenter, double-blind, placebo-controlled 18-month clinical trial of patients with heterozygous familial hypercholesterolemia (HeFH). Patients with established HeFH were taking a maximally tolerated dose of statin with or without other lipid-modifying therapy and required additional LDL-C reduction.
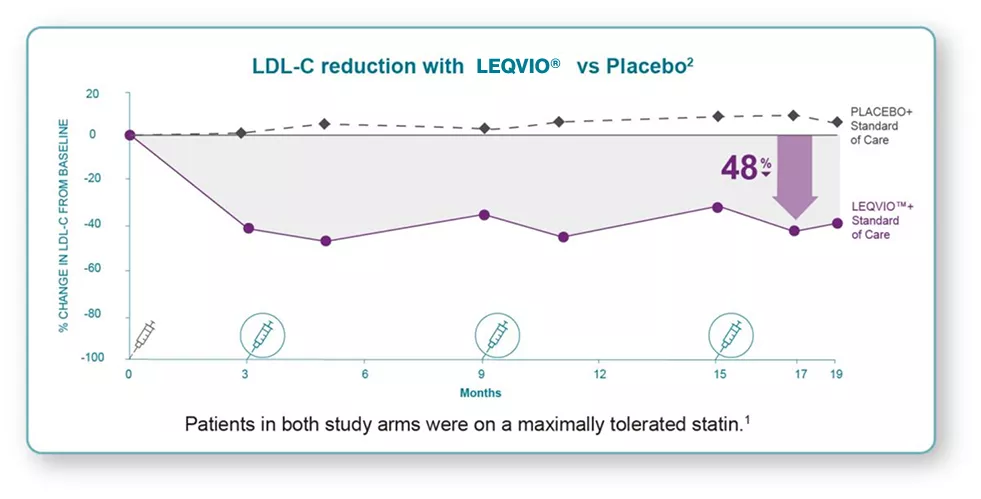
In ORION-9 clinical trial, LEQVIO® demonstrated2:
• 48% effective LDL-C reduction between-group difference at Month 17*
*Between-group difference of -47.9% (95% Cl: -53.5%, -42.3%; P<0.001) refers to the difference between the placebo group (8.2%) and the LEQVIO® group (-39.7%).
• 44% sustained LDL-C reduction between-group difference after Month 3 and up to Month 18†
†Between-group difference of -44.3% (95% Cl: -48.5%, -40.1%; P<0.001) refers to the difference between the placebo group (6.2%) and the LEQVIO® group (-38.1%).
Primary end point1: percentage change in LDL-C from baseline to 17 months compared with placebo and time-adjusted percentage change in LDL-C from baseline between 3 months and up to 18 months compared with placebo.
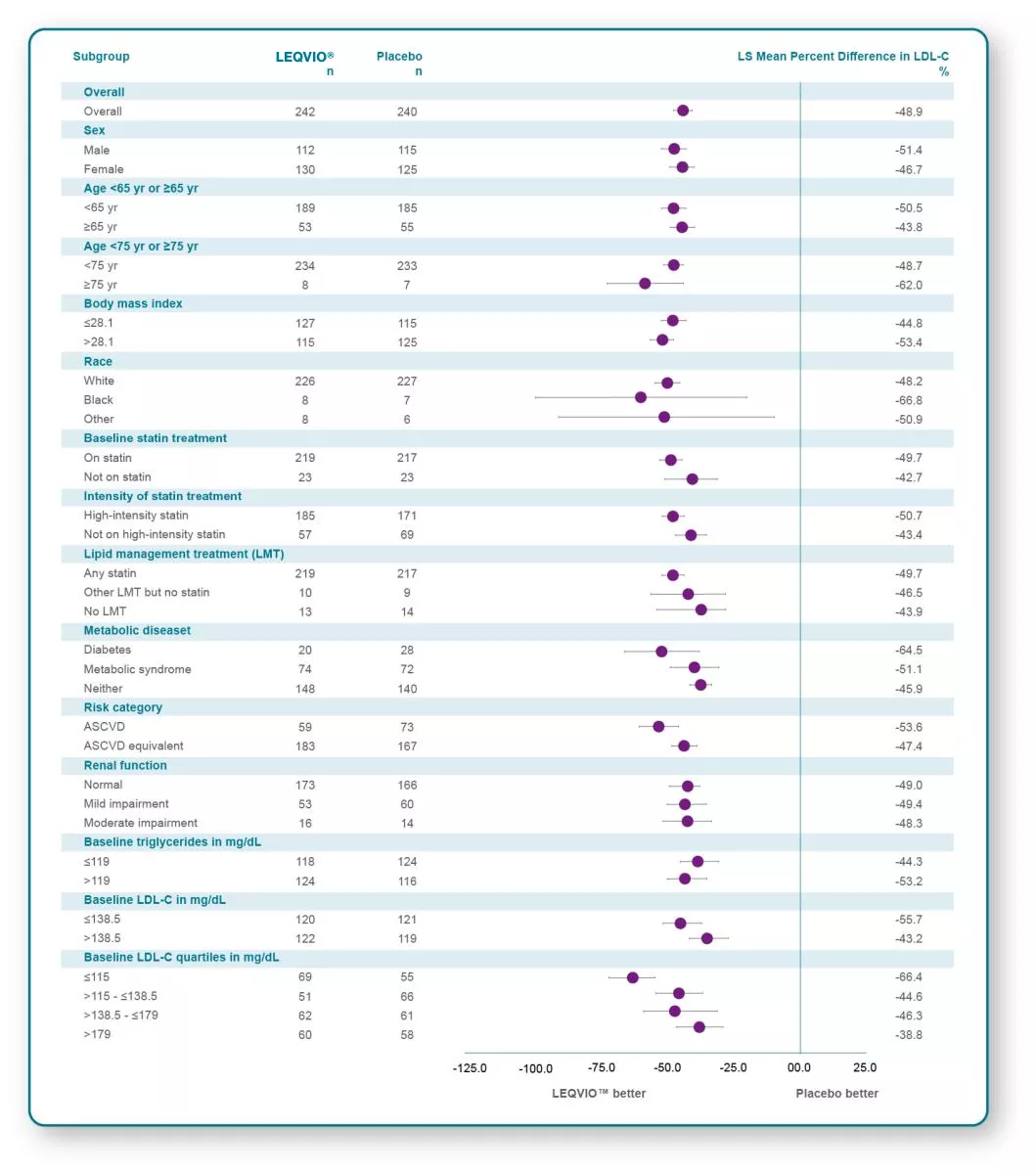
Reduction of LDL-C across prespecified subgroups in ORION-93
LEQVIO® provides significant, long-lasting reduction‡ of LDL-C across a wide spectrum of patient populations.3,4
‡LDL-C reduction was maintained during each 6-month dosing interval.1
Abbreviations:
ITT, Intent to treat; CI, Confidence interval.
References
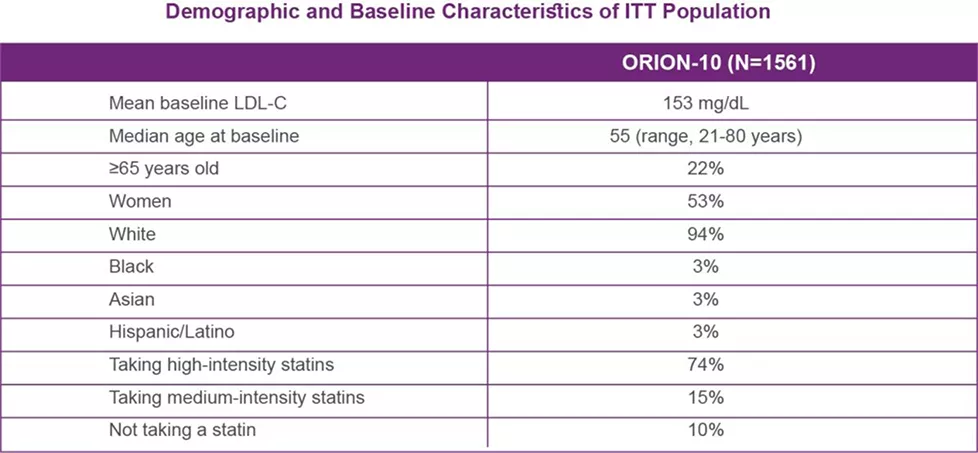
ORION-10: Efficacy in Atherosclerotic Cardiovascular Disease
Study Design1
ORION-10 was a multicenter, double-blind, randomized, placebo-controlled 18-month clinical trial of patients with established ASCVD were taking a maximally tolerated dose of statin with or without other lipid-modifying therapy and required additional LDL-C reduction.
The following data are focused only on ORION-10, as this study was conducted with patients with established ASCVD:
In ORION-10 clinical trial, LEQVIO® demonstrated:2
*Between-group difference of -52.3% (95% Cl: -55.7%, -48.8%; P<0.001) refers to the difference between the placebo group (1.0%) and the LEQVIO® group (-51.3%).
†Between-group difference of -53.8% (95% Cl: -56.2%, -51.3%; P<0.001) refers to the difference between the placebo group (2.5%) and the LEQVIO® group (-51.3%).
Primary end point3: percentage change in LDL-C from baseline to 17 months compared with placebo and time-adjusted percentage change in LDL-C from baseline between 3 months and up to 18 months compared with placebo
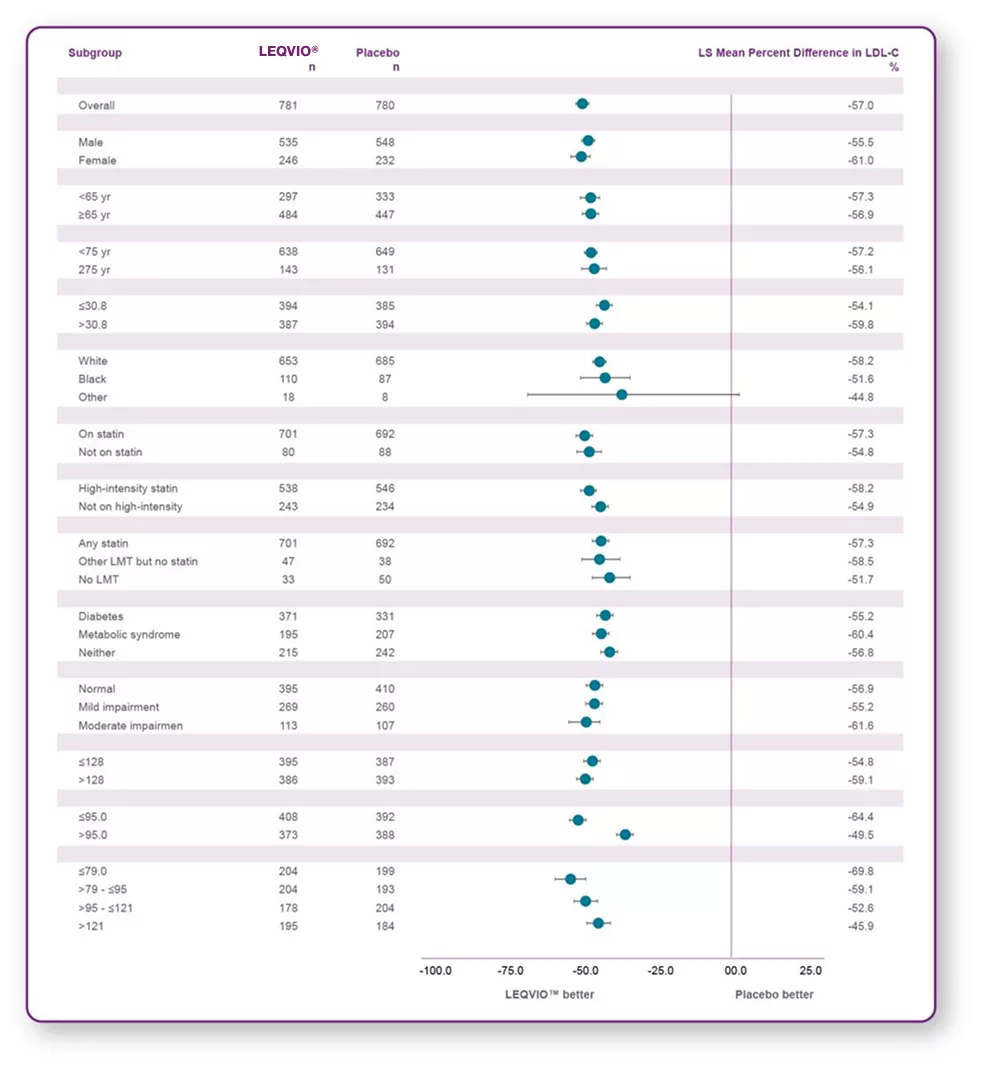
Reduction of LDL-C across prespecified subgroups in ORION-103
LEQVIO® provides significant, long-lasting reduction‡ of LDL-C across a wide spectrum of patient populations.3,4
‡LDL-C reduction was maintained during each 6-month dosing interval.1
Abbreviations:
ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; CI, Confidence interval.
References
LEQVIO® NSS - UAE
LEQVIO® NSS - UAE
